5/26/21 Steering Committee Meeting
We had another packed steering committee meeting with >35 participants.
The topics and all resources and content, along with a video, are provided below.
We look forward to hearing from you .
Watch the recording of the meeting above.
MDIC Updates 0:00-7:34
NODEX 7:34-13:35
NEJM Call for Submissions 13:36-17:38
Workgroup updates:
Payor 18:08-21:25
ML 21:32-22:55
Trainees 23:10-25:02
Data Standardization 25:29-26:32
Coding 27:00-29:20
Truthing & Validation 29:25-33:55
Pathology Informatics Summit Review 33:58-38:13
Crafting the PIcc concept 38:28-39:30
Project updates
TILS RS 39:37-42:54
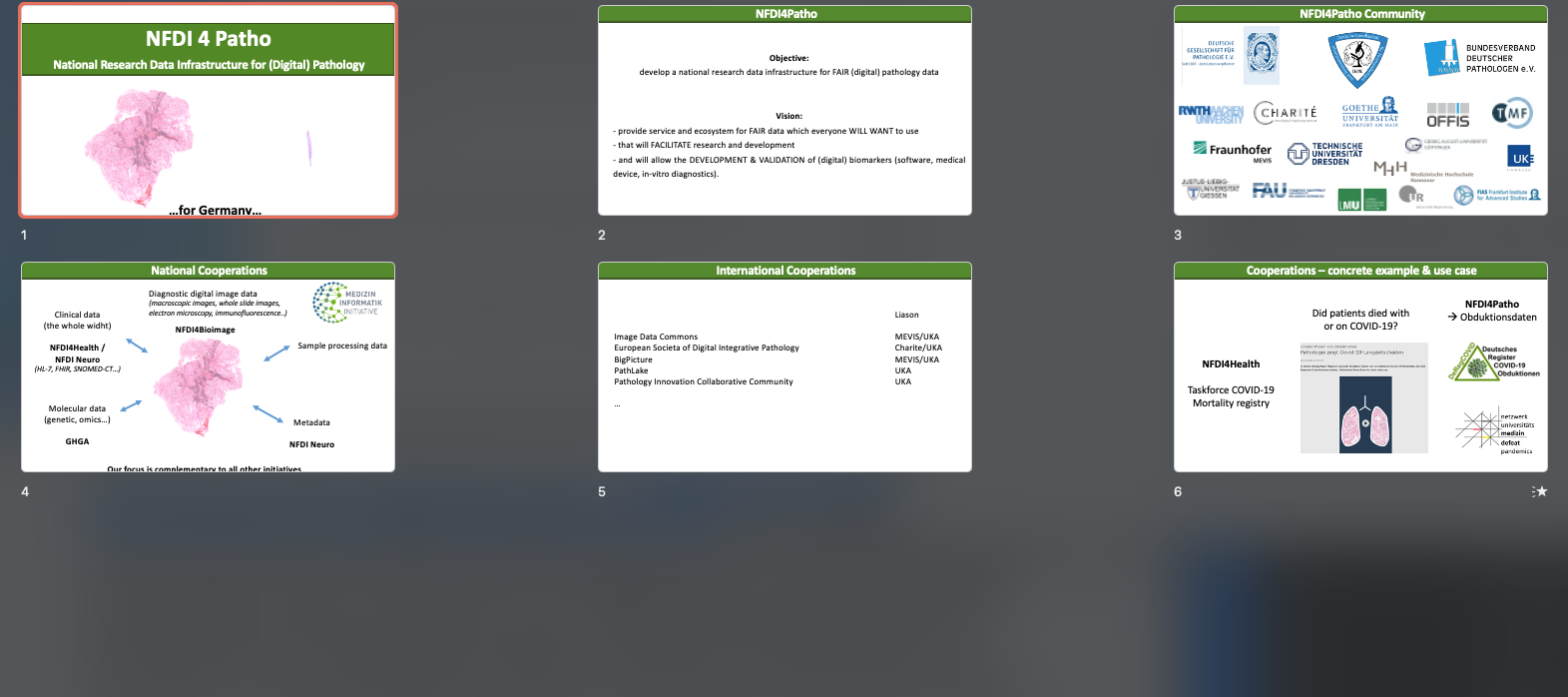
NFDI4PATHO 43:10-51:10
Reimbursement 51:19-51:45
FOCR Update 51:50-53:50
Additional Updates 53:51-end
Meeting Summary
MDIC Annual Public Forum:
Collaborative Communities: Putting the community in the driver’s seat to navigate healthcare challenges (link)
Artificial Intelligence powered cancer diagnostics: How regulatory science can re-shape digital diagnostics
Joe Lennerz (Introduction scope and vision), Ed Margerrison (FDA; Regulatory science), Matthew Hanna (AI powered cancer diagnostics). Markus Herrmann (Interoperability challenges), Laura Lasiter (Patient advocacy perspective PIcc), Esther Abels (Payor Workgroup Updates)
The Annual Public Forum is open to the public to highlight high profile initiatives actively working with the FDA.
Network of Digital Health Experts
Link HERE.
How to become a member: if you are affiliated with an association that is one of the network’s partner organizations, you can individually contribute.
NEJM Call for submissions:
Click HERE to download the NEJM Catalyst AI Theme Issue
Workgroup Updates:
Payor
Conversation with AMA during bi-weekly meeting:
Think about how radiology moved forward with establishing CPT codes and if we can use that for pathology
Reach out to other expert groups in this field to develop a more comprehensive plan and strategy on how CPT codes can be planned and structured
ML
Recent discussion on what deliverables the group should be focusing on. Landed on: Create a rubric tool and/or decision tree for helping people as they start to design experiments for validation so the key concerns that we would have from an analytical standpoint get answered in the correct way
Collaborated with the Trainees group for a “wishlist” as a series of use cases to help us walk through the problem and make sure the rubric/decision tree meets them
Trainees
In collaboration with the ML group, PIccTG hosted a brainstorming session on potential use cases for testing of AI algorithm in development
List available HERE
Data Standardization
Progress working on primary goal of establishing a repository of standard data sets through DICOM WG26
DICOM Hackathon ongoing
Truthing and Validation
Pathology Informatics
Brandon Gallas gave a live presentation at the 2021 Pathology Informatics Summit. Link HERE
FDA Science Forum
HTT project has a poster hosted through the 2021 FDA Science Forum. Link HERE
Summer data collection on the microscope:
Two data collection host sites: Yale (Kim Blenman) and Stony Brook (Joel Saltz)
Readying a recruitment document to encourage people in the area to participate
Preparing GitHub repository with pathologist evaluations
Purpose: Share and support development of analysis methods
Pathologist-pathologist aggrement is baseline for algorithm-pathologist agreement
Walk through HTT team
Burroughs Welcome Fund
Purpose: Support slide sourcing and data-collection platform development
Passed Stage 1
Stage 2 interview June 9
HTT Related: Google Summer of Code projects
Link HERE
Project [7] Modularize the current code base for quick and easy workflow / task customization
Project [8] Integrate an optical microscope with a camera and motorized stage
Pathology Informatics Summit Review
Link HERE
Project updates:
TILS RS [Kim Blenman]: Looking for collaboration from patient advocacy and pharmaceutical representatives.
NFDI4PATHO [Peter Boor]: national research data infrastructure that would cover pathology and digital pathology focusing on verification of data. We would like to engage in a collaboration and ask PIcc to provide a letter of support that would help with their funding application.
Reimbursement [Esther Abels]: plans to have some data to present at next meeting
Crafting the PIcc statement:
FOCR Update:
Thank you to Laura Lasiter for introducing the new FOCR representative for PIcc, Brittany McKelvey.
General Updates:
Publications
FDA Science Forum (May 26-27) happening now (link)
Discuss possible Annual meeting near DPA Visions (October 17-19 in Las Vegas, NV)
Updated landing page with logos – now live