12/22/21 Steering Committee Meeting
We had a great final steering committee meeting of the year.
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
Watch the recording of the meeting above.
0:00-3:20 Introduction
3:21-10:24 National Tokenization Mechanism
10:25-19:24 CLIA Waiver Project
19:25-20:49 LDT vs. IVDR
20:50-24:23 TILs Project
24:23-35:04 Noteworthy Papers
35:05-51:00 Quarterly presentations + annual meeting discussion
51:01-end Closing remarks
Meeting Summary
National Tokenization Mechanism
Convergence of two prior projects, Privacy Pilot Project and Information Blocking
Panel proposal submitted to ISPOR
Learn more here
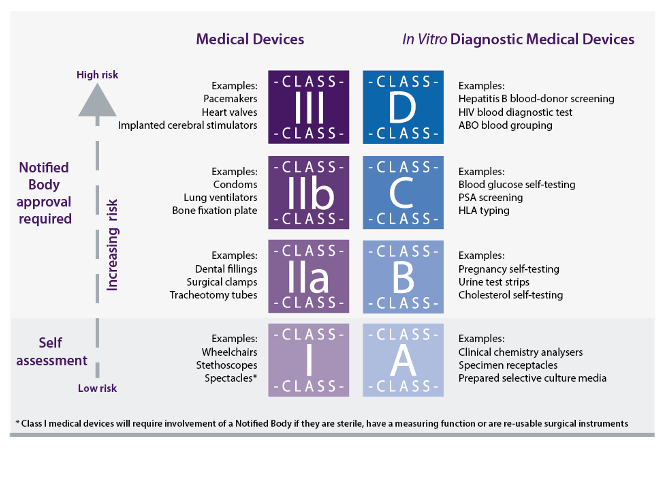
“CLIA Waiver” for Digital Pathology
State survey agency contact
Draft letter to CMS in progress
Follow-up: LDT vs. IVDR project
More information following proposal
Reach out to digipathalliance@gmail.com if there are topics you are interested in discussing
TILs Project
Biomarker Terminology: Speaking the Same Language from FDA Biomarker Qualification Program (link)
Survey for professionals and a survey for patient advocates to gain perspective on what is needed for putting forth a biomarker that is morphology based
Noteworthy Papers
Bélisle-Pipon et al., What Makes Artificial Intelligence Exceptional in Health Technology Assessment (link)
Sheinson et al., Trends in Use of Next-Generation Sequencing in Patients With Solid Tumors by Race and Ethnicity After Implementation of the Medicare National Coverage Determination (link)
Sammut et al., Multi-omic machine learning predictor of breast cancer therapy response (link)
Elemento et al., Artificial intelligence in cancer research, diagnosis and therapy (link)
Kim et al., Hidden in Plain Sight: Clinical Informaticians are the Oncology Subspecialists You Did Not Know You Needed (link)
Quarterly Presentations
Publication on payor strategies
MDDT submission
Campaign to recruit at least 2 more patient advocacy group
Annual PIcc meeting (in-person)?
Annual Meeting
USCAP in March (Los Angeles) = socializing event
API in May 9th-12th 2022 (Pittsburgh) => possibility
Proposal for a session on regulatory science (January 31st ?)
Data gathering “Sunday before”? (FDA)
FDA proposal for HTT project already submitted
CAP2022 in October (New Orleans)
Resident forum => “get the word out” to trainees
Data gathering ?
ASCP meeting
Data gathering session