4/27/22 Steering Committee Meeting
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
Watch the recording of the meeting above.
0:00-2:45 Introduction
2:46-9:45 CLIAC Meeting
9:46-18:07 Additional Updates
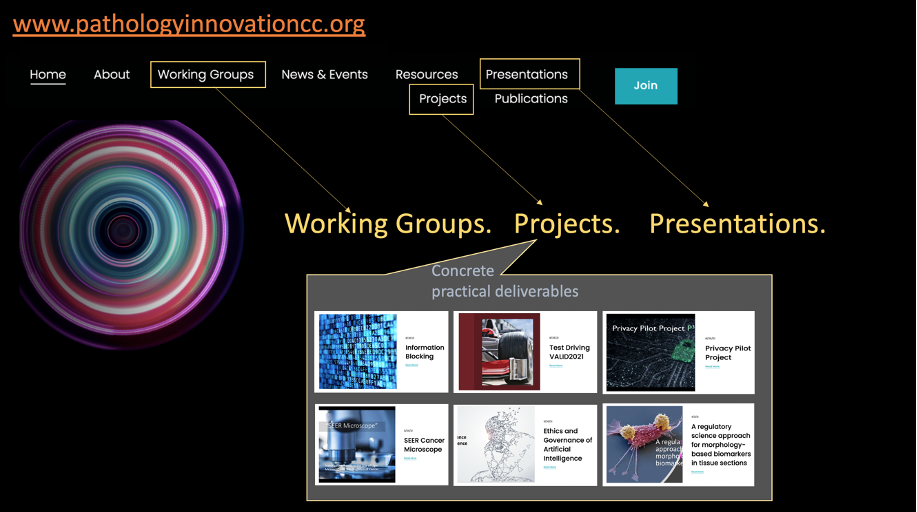
18:08-22:00 PIcc MS Teams Channel
22:01-24:50 Review of org outline
24:51-28:05 Patient advocacy
28:06-34:06 EMA Corner
34:07-35:01 FDA
35:02-38:22 Featured papers
38:23-42:03 Resources
42:04-44:45 News & Events
44:46-end Discussion
Meeting Summary
Clinical Laboratory Improvement Advisory Committee Meeting
numerous public comments were submitted
API Public Comment April 2022 (download)
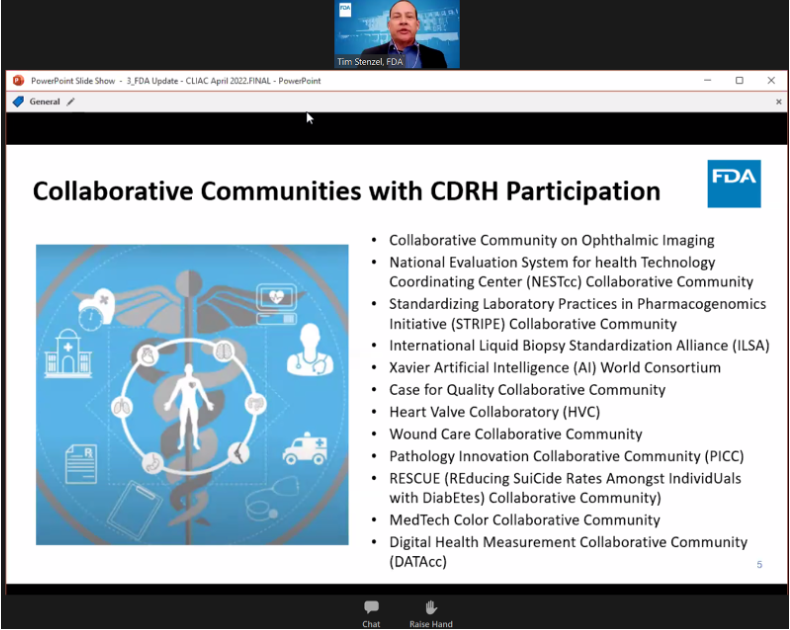
Tim Stenzel, FDA director of Office of Health Technology 7 (OHT 7: In Vitro Diagnostics and Radiological Health - OIR) presented (among may topics: Collaborative Communities and PIcc)
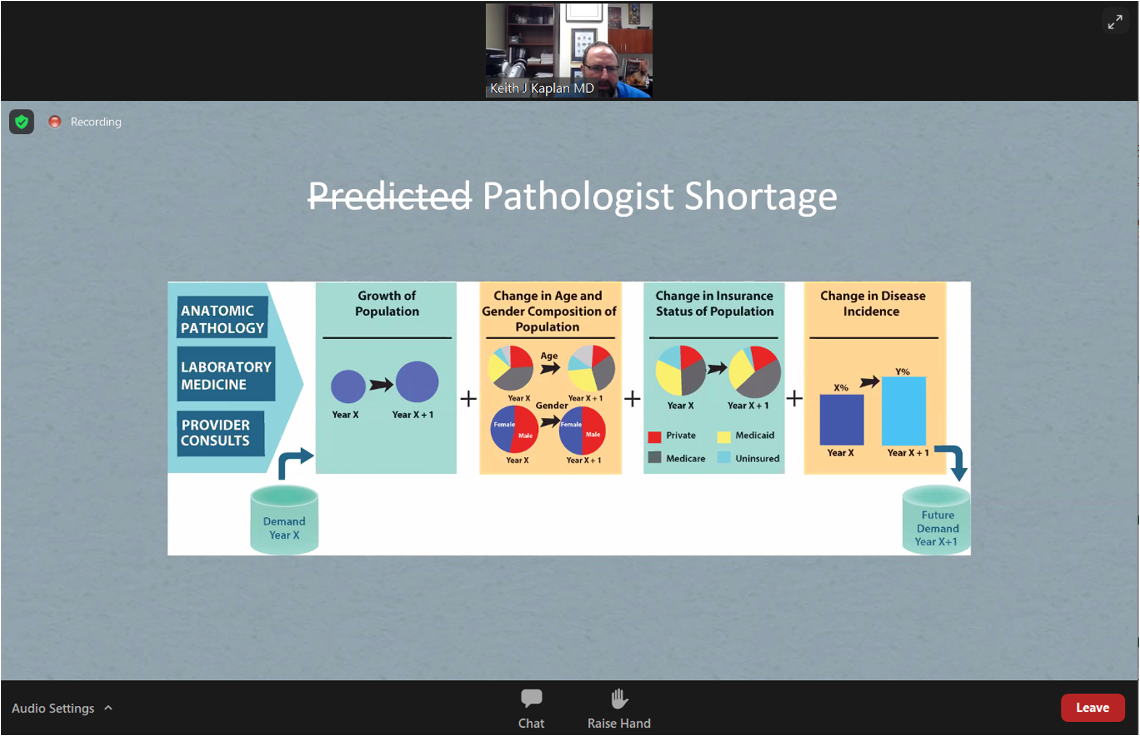
Keith Kaplan presented “Digital Pathology: The Past, Present, and Future”
still awaiting official recommendation
CLIA Waiver project - please reach out if you’d like to contribute
Additional Updates
request by community to host a session that covers the decision summary from PAIGE Prostate
currently collaborating with PAIGE regulatory team
will announce session date when it is set
AI Grand Challenges discussion session took place on 4/25, watch the session here
follow up session date TBD
AACR New Orleans took place
Kate E: suggestion for PIcc business cards, lots of interest in digital pathology
AACR had a special topics meeting in 2021 on AI in Diagnosis and Imaging and I think it was it's first dedicated meeting touching on digital pathology (link)
Keith W: how much is AACR thinking about digital pathology?
DeciBio (link)
Joe S: AACR companion meeting at USCAP (link)
MS Teams Channel
PIcc channel for direct file exchange with FDA
Project and workgroup progress GANTT chart to be created
call for assistance from members with web-dev skills
Review of organization of the community
Patient Advocacy
Friends of Cancer Research Annual Report 2021 (download)
APPIA collaboration pending
protocol development for assessing pre-analytical variability proposed as a collaborative project
Sepsis Alliance
multi-system disease poses an interesting regulatory question
help to form collaborative community
EMA Corner ‘Out of the Box’
ICH guideline Q14: Intended for analytical procedure development (download)
Interesting perspective: risk based assessment is well established in the analytical performance field when it comes to drug manufacturing controls, could be applied to something we relate to
Joe S.: write a paper to translate these guidelines into pathology/molecular space = “Rosetta Stone”
FDA Guidance document for products between “drug” and “device”
Ophthalmic products (download)
things that can be learned from these specific use cases
Featured papers
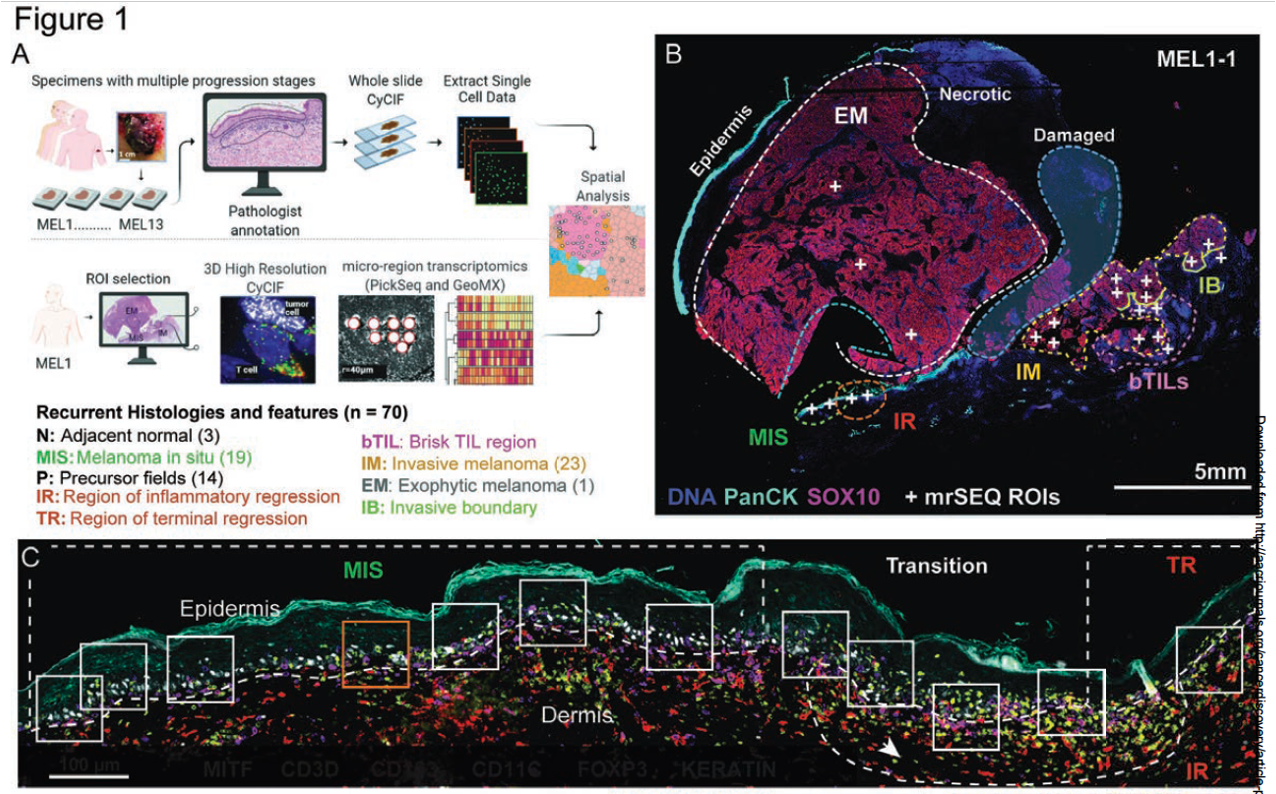
Nirmal et al., The spatial landscape of immunoediting in primary melanoma at single cell resolution (download)
Bhate et al., Tissue schematics map the specialization of immune tissue motifs and their appropriation by tumors (download)
Vasudevan et al., Digital biomarkers: Convergence of digital health technologies and biomarkers(download)
Moses and Pachter. Museum of spatial transcriptomics(download)
Nurk et al., Complete sequence of the human genome (3/31/2022) (download)
Resources
Python ML Guide (download)
Report 2022: Clinicians of the future (download)
Chapter 3 - The Future Tech-Savvy Clinician
Bruce Quinn 2022: Cancer Patient and Access to Molecular Diagnostics (download)
HAL: Nicolas Rougier, Scientific Visualization (download)
News & Events
May 9-12 Pathology Informatics Summit 2022 Pittsburgh
PIcc events 5/10 and 5/11 at a TBD location
June 3-7 ASCO meeting Chicago; Question => joint (formal) event?
June 6 at 10-11 AM ET PathML: an open-source software toolkit for computational pathology research (link)
May 25 at 3-4 PM ET Next steering committee meeting (link)
Discussion