REAL-AIM
Building Regulatory-Ready Datasets: A Collaborative Approach for Validating AI Models in Breast Cancer Diagnosis
◦ Initial proposal was shared with FNIH BC-CSC co-chairs in May, which will go under a 2-phase review: concept (overall idea and plan (granular details).
◦ Feedback from the co-chairs was very positive and recommended a narrow-focused project plan to show value added and proof of concept.
◦ Next step is to present concept plan to the FNIH BC-CSC steering committee for feedback, support, and alignment with FNIH members.
◦ Shooting for July 31st Cancer Steering Committee meeting
Background
Emergence of the project idea (PIcc “vote”)
The initial idea was derived collaboratively from within the membership of PIcc. The group convened briefly during the annual meeting on June 27-28, 2023 to meet, synergize, and impact. Link to meeting: https://pathologyinnovationcc.org/picc23-annual-meeting
All presentations from the meeting as well as the program are available here: https://pathologyinnovationcc.org/meeting-summaries/031hwexwnb56twp82eqktehfl6ba6v
One declared goal from the group was to derive one (n=1) key project that the community values the most. The topics were determined before the meeting and included Remote Work, PCCP, Statistics, and the final breakout session included an audience-solicited topic. The voting took place, and the selected topic was “Regulatory pathway for real-world data collection and evaluation methodologies for AI-driven devices”.
The SWOT charts for all topics are available here: https://pathologyinnovationcc.org/meeting-summaries/031hwexwnb56twp82eqktehfl6ba6v
About the FNIH
The Foundation for the National Institutes of Health (FNIH) is a not-for-profit, 501(c)(3) charitable organization established by the US Congress in 1990. Located in North Bethesda, MD, the FNIH raises private-sector funds, and creates and manages alliances with public and private institutions in support of the mission of the National Institutes of Health (NIH).
FNIH, Biomarkers Consortium, Cancer Steering Committee
The Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium Cancer Steering Committee (BC-CSC) annually convenes more than 200 experts from academia, pharmaceutical companies, biotechnology companies, not-for-profit organizations, the National Cancer Institute (NCI), the U.S. Food and Drug Administration (FDA) and other federal agencies to focus on biomarker discovery and development across the cancer landscape. The Symposium offers a unique opportunity to prioritize and strategize future directions in biomarker discovery and development. Attendees have an opportunity to explore cutting-edge science, hear feature presentations from experts, brainstorm critical areas of biomarker research need and pitch collaborations designed to inform the field of exciting new advances.
Initial contact with FNIH was established in the summer of 2023 and the first meeting was in August 2023
FNIH Cancer Steering Committee Meeting November 14th, 2023
To ensure alignment with the selected topic (Regulatory pathway for real world data collection) as well as the scope and vision of the cancer steering committee we selected 3 initial use cases.
PIcc developed an outline for the presentation at FNIH
The meeting was a success and in early 2024 we worked on the creation of an initial draft of the PIcc FNIH Proposal
On April 12th 2024 we submitted an initial version to FNIH and received feedback on April 22nd 2024 and scheduled a follow-up call for May 3rd 2024
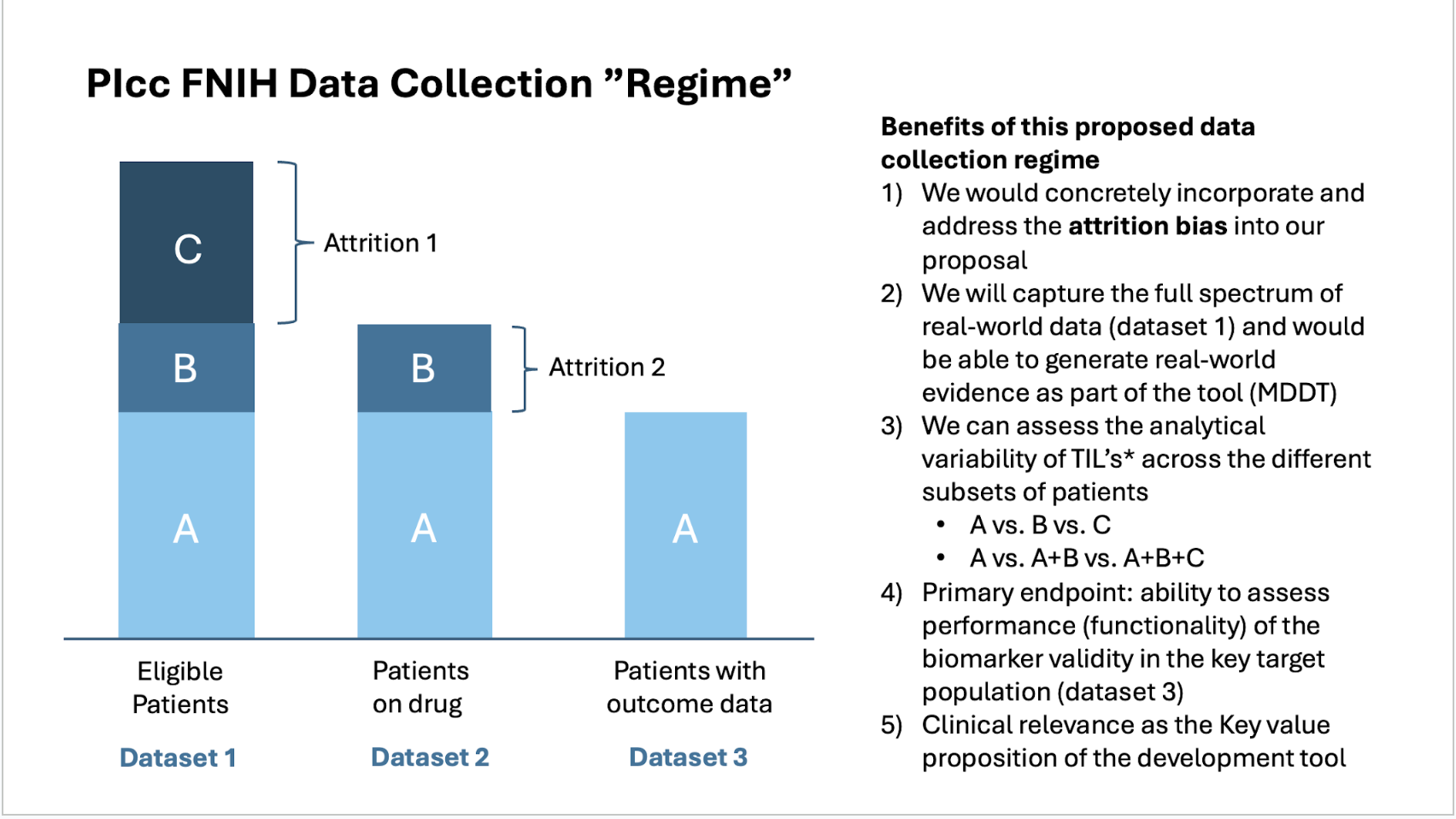
We aimed to incorporate the revisions for a review by the cancer steering committee at the end of July 2024. And part of this presentation was the PIcc FNIH Data Collection Regime
A summary of the project thus far was provided during the May 2024 Plcc Steering Committee meeting (recording: topic begins at 17:38).
The cancer steering committee met on July 31st 2024 and their assessment was that the project need to be developed further
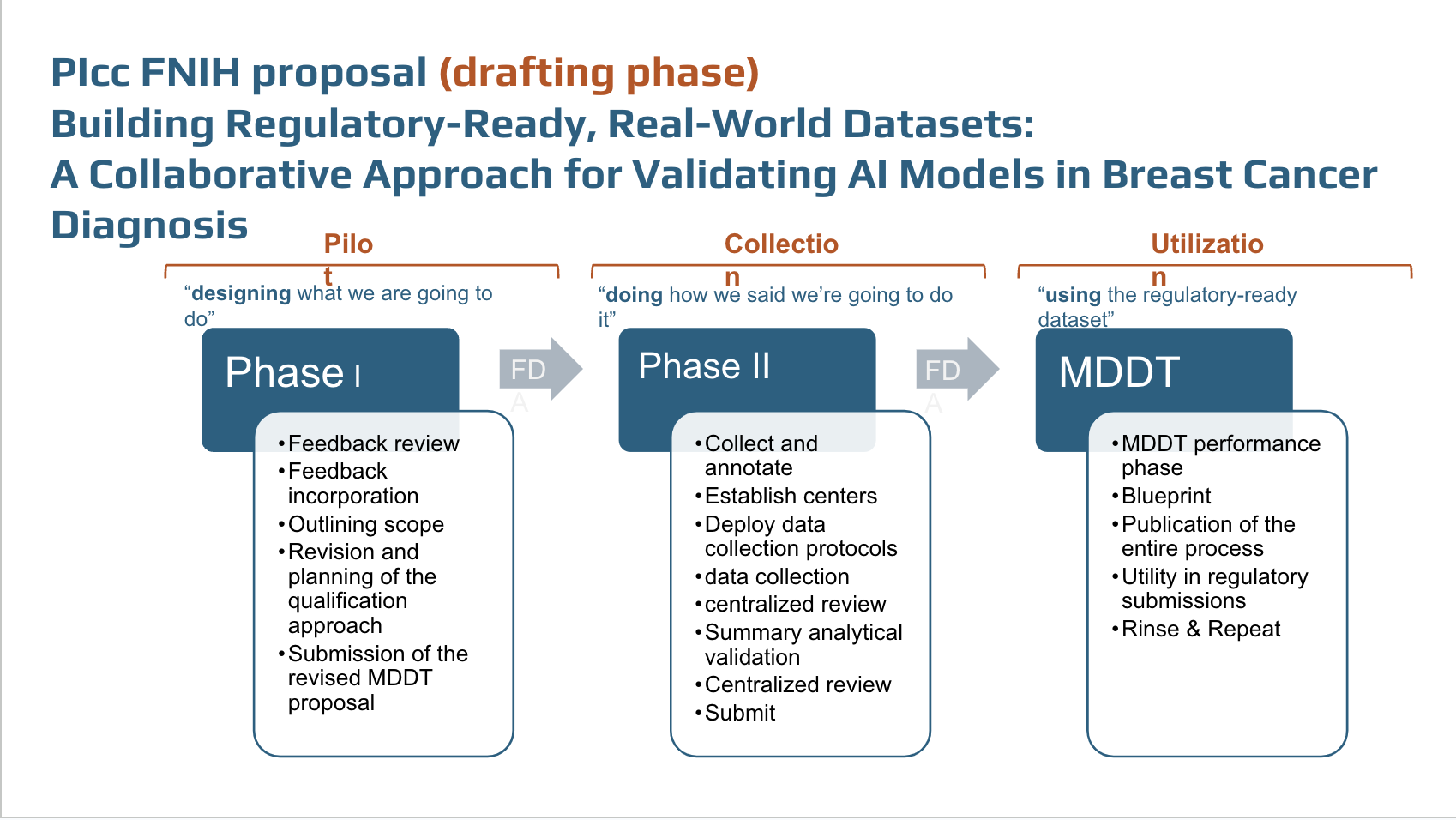
During the months of August-November 2024, we solicited additional feedback from the community and derived a specific plan regarding the project phases with a pilot, a collection phase, and utilization phase.
Authors
Alex Karargyris PhD, ML Commons, Co-Chair for Medical working group
Joe Lennerz MD PhD, ‘Chair’ Pathology Innovation Collaborative Community (PIcc), Chief Scientific Officer, BostonGene, Waltham, MA
Brandon D. Gallas, PhD, FDA, CDRH, OSEL, Division of Imaging, Diagnostics, and Software Reliability, Federal Liaison PIcc, Principal Investigator HTT Project
Alex Kalof, MD, President-Elect Association for Directors of Surgical Pathology, University of Vermont
Matt Leavitt, MD PhD, DDx Foundation
Brittany McKelvey PhD, Friends of Cancer Research
M. E. de Baca, MD, Vice President of Medical Affairs, Sysmex America, Inc., Chair, College of American Pathologists Council on Informatics and Pathology Innovation
Micah Sheller PhD, Intel / MLCommonsLaura Lasiter PhD, AstraZeneca
Kim RM Blenman PhD, MS, Yale University, Assistant Professor of Medicine (Medical Oncology) (School of Medicine), Assistant Professor of Computer Science (School of Engineering and Applied Science)
Amy Ly MD, Massachusetts General Hospital, Associate Professor of Pathology, Harvard Medical School, Boston, MA
Carl Barrett MD PhD, UNC
Emma Gardecki, FDA