7/27/22 Steering Committee Meeting
Please find the topics, all resources and content, along with a video recording of the meeting below.
We look forward to hearing from you.
Watch the recording of the meeting above.
0:00-2:56 Intro
2:57-5:13 Telehealth
5:14-7:38 MDUFA/VALID
7:39-9:15 PIcc Charter
9:16-16:28 FDA Corner
16:29-24:06 New & Updates
24:07-32:12 Resources
32:13-36:57 Project updates
36:58-54:05 Featured papers
54:06-end Closing remarks
Meeting Summary
Advancing Telehealth Beyond COVID–19 Act of 2022
Removing geographic requirements
Clear indication and alleviates national concerns
Is expected to pass the house and will result in a 2-year extension
Devices + diagnostics is not mentioned (remains TBD)
MDUFA/VALID – FDA oversight of LDT’s
CAP provided an update via a webinar on of LDTs and the VALID Act
VALID Act support letter from CAP, ASCO, FOCR, Pew, and more (download)
AMP, AACC, ASCP, API & many more letter (download)
Statnews article (Sen. Burr press release)
PIcc Charter
We have received questions about how to join
Free, available, charter can be signed à symbol on the landing page and participation
Non-profits 501(c)(3) can join
Proposal to start a non-profit organization as a facilitator
FDA Corner
Technical Performance Assessment of Quantitative Imaging in Radiological Device Premarket Submissions (download)
Conducting Remote Regulatory Assessments Questions and Answers Draft Guidance for Industry (download)
FDA Details Optimized Approach for Regulatory Oversight Tools to Better Protect Public Health (link)
FDA’s Ongoing Use of Inspectional Tools for Ensuring Access to Safe, Quality Food and Medical Products During the COVID-19 Pandemic (link)
Analysis of User Fee metrics
Catalog of Regulatory Science tools (link)
FDA Drug safety podcasts (link)
Example: Ukoniq (umbralisib; PIK3CA inhibitor) withdrawal (link)
Update CDRH’s Electronic Medical Reporting (eMDR) System Enhancements (link)
Example: Coding resources for medical device reports
Adverse Event Codes (link)
Proposed Rule on Revising the National Drug Code Format (link)
Two special events
Brandon Gallas PhD; PIcc Liason agreed to 2 seminars (link)
8/5/2022; 12:00 EST: Title: “Evaluating Medical Imaging Devices and Image-Based Algorithms with the Clinician In The Loop”
TBD: 2-hour online workshop: “Assessing Agreement and Reader Reliability in Medical Imaging Analysis: A Redux of a session from the 2023 Joint Statistical Meeting.”
News & Updates
Truthing & Validation WG updates (Brandon Gallas PhD, FDA) (link) (download)
Legislative Update: Food Safety Administration Act of 2022 (link)
Baby formula supply shortage
Proposes to separate “food” oversight from the FDA
New enforcement entity would be a separate branch under HHS
Relevance: drug development relies on intra-agency experience
Fabrication in scores of Alzheimer’s articles, threatening a reigning theory of the disease (link)
Image-based assessment of data fabrication
Regulatory science relevance: e.g., drug development
AI advancements in Sepsis diagnostics
Resources
MDCG 2019-11 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR
Clarifies EU decision steps to qualify MDSW (=US term SAMD)
IMDRF Machine Learning-enabled Medical Devices: Key Terms and Definitions (link) including “Introduction to Online ML” (link)
DATAcc Toolkit for Inclusive Deployment, Digital Medicine Society (link)
Prodigy: Radically efficient machine teaching. An annotation tool powered by active learning (link)
Big problem in data science and ML/AI: data annotation
Radically new approach to optimize annotation (text and image)
Very user friendly; and disruptive
WHO Breast Cancer Initiative (link)
New tool for capturing the full Care-cycle of breast cancer patients
Pre-diagnostic and diagnostic pillar to a four tiered cycle
Project updates
FDA cleared comments for Decision summary session (presentation June 2022) are up (link)
HER2-low (NEJM paper + ASCO, now change in paradigm of breast cancer testing)
Abemaciclib/Ki-67 in breast cancer project
ctDNA out of the dark session (link)
Friends hosted two sessions on the topic
review of draft guidance is up
received first comments
Featured Papers
Ochoa et al., Human Genetic findings support 2/3rds of the 2021 FDA approved drugs (link)
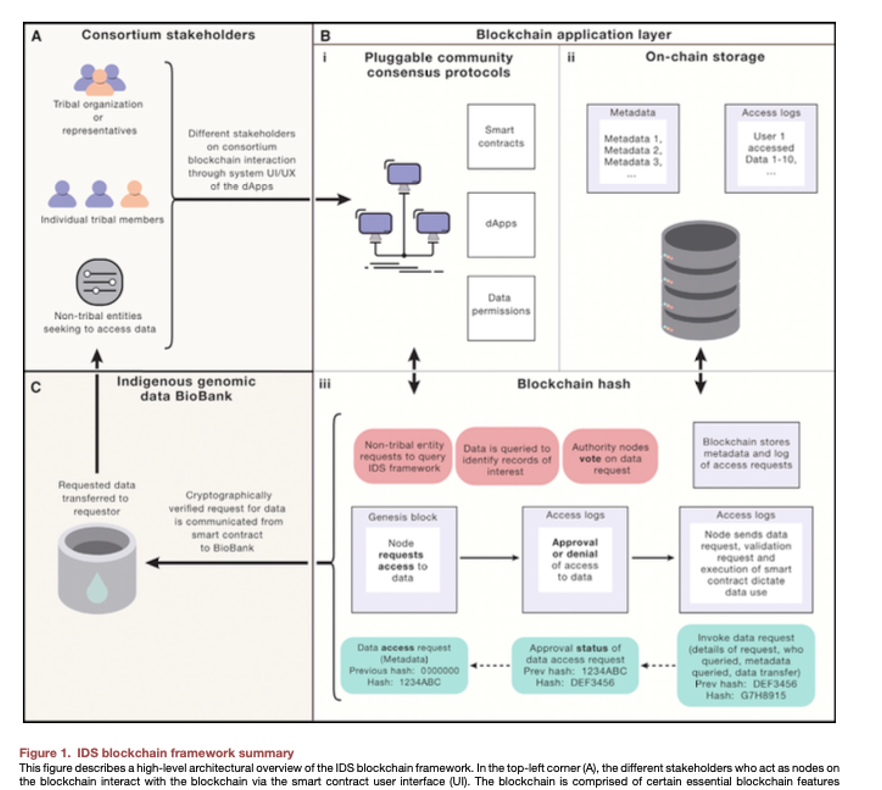
Mackey et al. Establishing a blockchain-enable indigenous data sovereignty framework for genomic data (download)
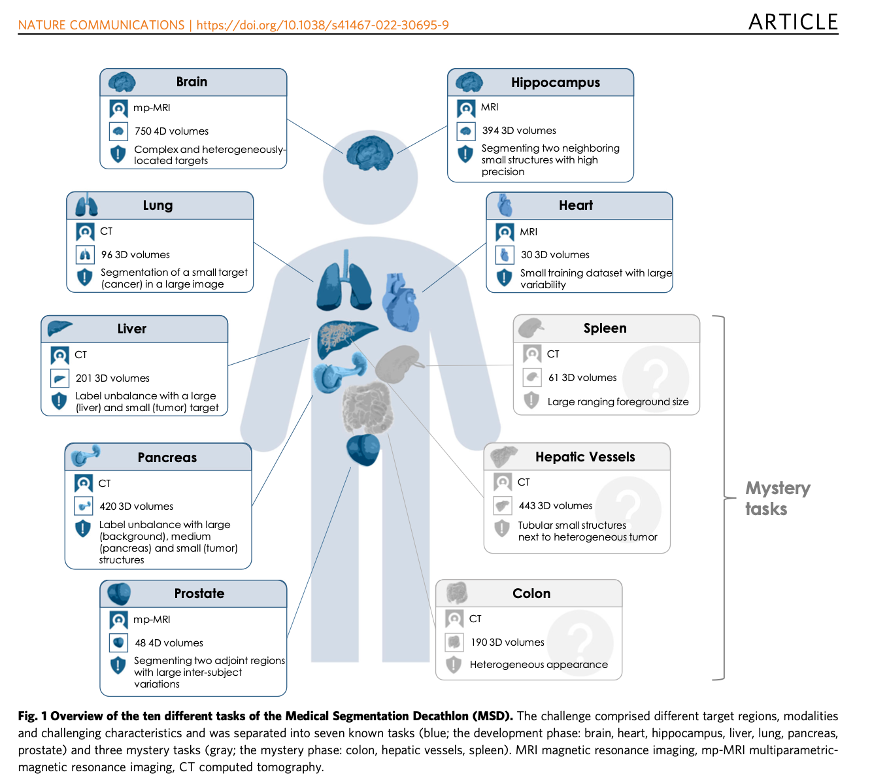
Antonelli et al. The Medical Segmentation Decathlon (download)
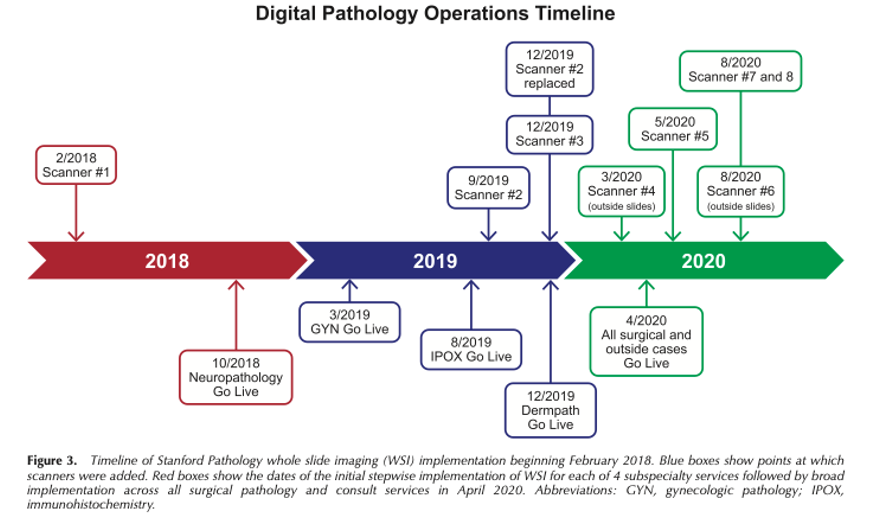
Rojansky et al. Rapid Deployment of Whole Slide Imaging for Primary Diagnosis in Surgical Pathology at Stanford Medicine (download)
Kelly et al. Job Stress, Burnout, Work-Life Balance, Well-Being, and Job Satisfaction Among Pathology Residents and Fellows (download)
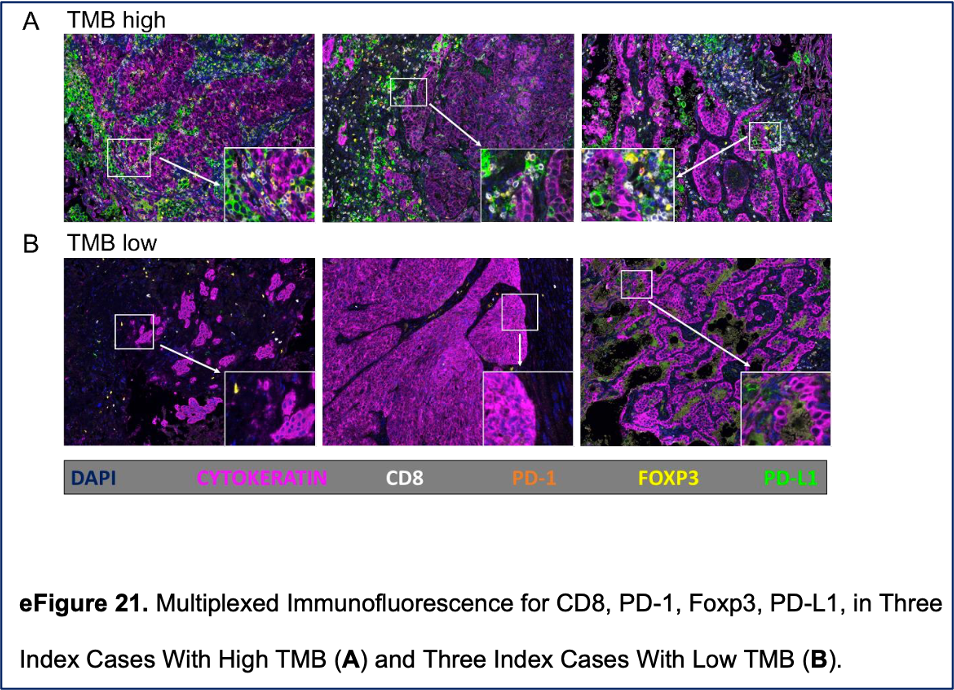
Ricciuti et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers with Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels (download)
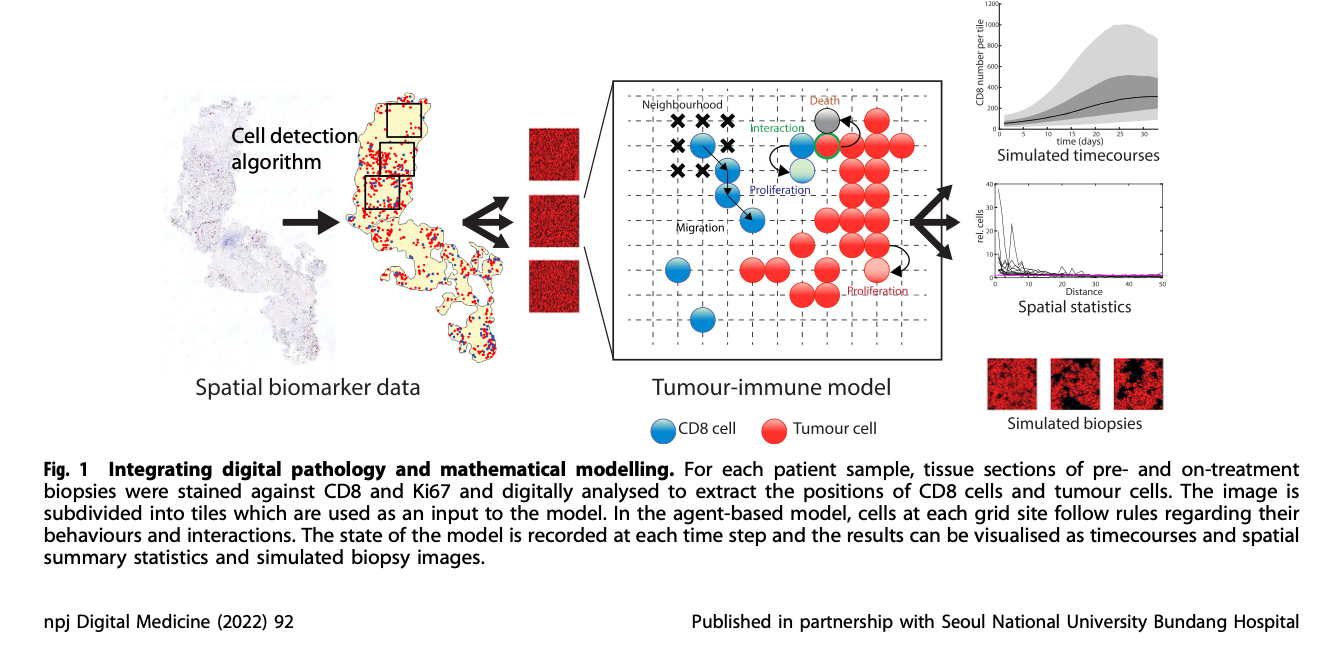
Hutchinson & Grimm. Integrating digital pathology and mathematical modeling to predict spatial biomarker dynamics in cancer immunotherapy (link) (download)
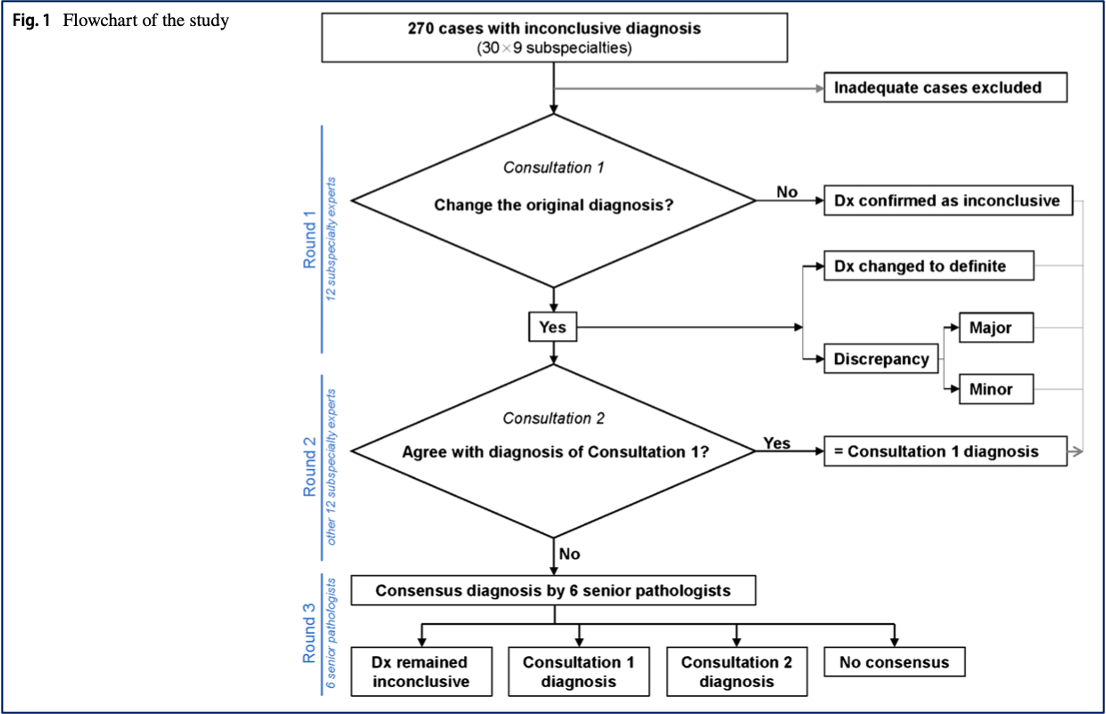
Shinohara et al. Substantial improvement of histopathological diagnosis by whole-slide image-based remote consultation (link) (download)
Hassell et al. Pathology education powered by virtual and digital transformation (download)
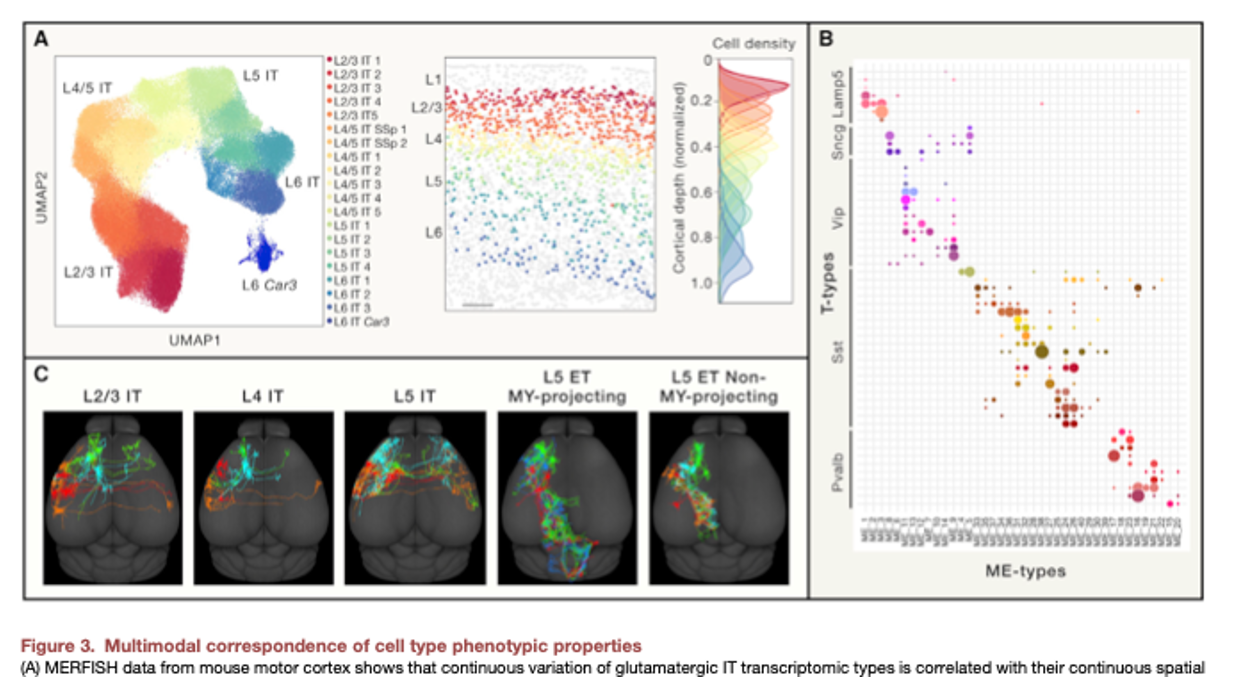
Hongkui Zeng What is a cell type and how to define it? (download)
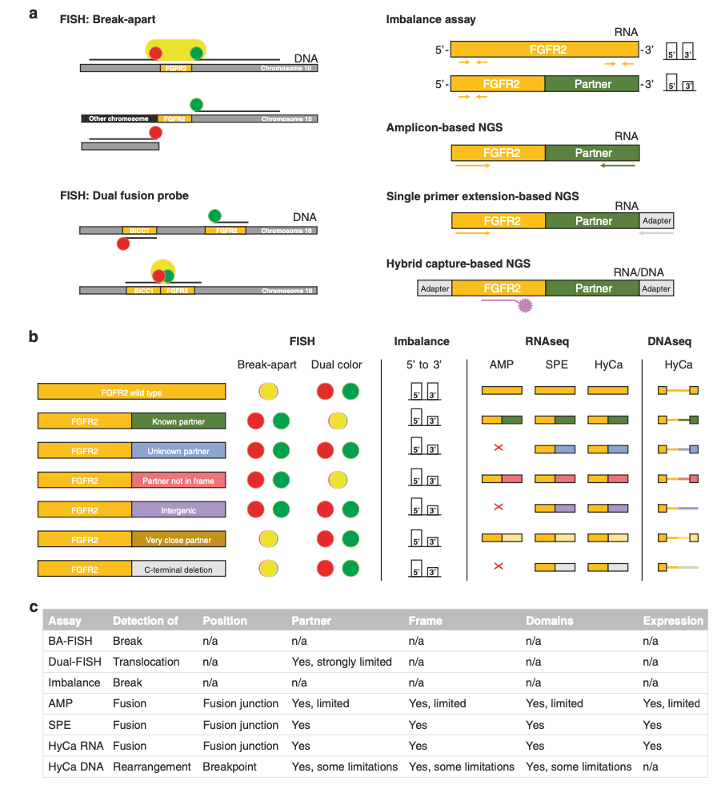
Neumann et al. Genomic architecture of FGFR2 fusions in cholangiocarcinoma and its implication for molecular testing (download)
Additional resources
Book: A Practical Guide for Health Researchers (download)
American Association for Clinical Chemistry: Point-of-Care Testing: A “How-To” guide for the non-laboratorian (download)
Tissue-Based Pathology Companion Diagnostic Development for Regulated Applications (download)
A standard for standards – principles of standardization (download)