8/31/22 Steering Committee Meeting
Due to technical Issues, there is not a recording of the August Steering Committee Meeting.
Meeting Summary
Digital Pathology CPT Codes
Category III codes = tracking
Clinical Utilization can help facilitate Medicare in establishing national reimbursement rates.
New codes (download)
Estimated to be ~3-5% of the global rates for existing codes
Could be ~$2-4
Prepare Systems to Report
Go-live is January 1, 2023
FDA Updates
FDA Modernization in Action 2022 (download)
FDA Modernization Framework (download image)
Dr. Gallas Lecture “Evaluating Medical Imaging Devices and Image-Based algorithms with the Clinician in the Loop”
August 5th
online now (link)
Multi-Reader Multi-Case Resource
Office of Global Policy and Strategy
FDA rumor control (link)
FDA approves first targeted Therapy for HER2-Low Breast Cancer
Legislative Update
Biomarker Testing: Health Insurers Will Be Required to Cover Cancer Testing in RI (download)
Go live: Jan 1, 2024
Rhode Island Genetic Counselor Licensing Act (download)
GCs are licensed but Licensure is not recognized
Access to Genetic Services Act
NSGC
CMS to recognized GC as independent providers
Coalition of supporters (>350 organizations)
Bipartisan support
End of the year Medicare package ??
96040 (CPT)
Biden Cancer Moonshot Relaunch Will “End Cancer as we know it” (link)
Biden Memorandum: “Ensuring Free, Immediate, and Equitable Access to Federally Funded Research” (download)
Peer Review “The New Peer Review: Why ‘Unbiased’ Science is Often Misleading” (link
A. VALID/FDASLA/MDUFA
Currently most societies wait to hear what might happen...
With Congress in recess, things are relatively quiet,
Staffers are probably working behind the scenes on reconciliation.
The week after Labor Day a mad scramble will begin to get MDUFA over the finish line (with or without VALID)
FDA has indicated they’ll need to start sending out furlough warnings if FDASLA [pewtrusts.org] doesn’t pass by the end of September
The FDA probably has enough funding until November/December = that sets the timeline
B. SALSA
The SALSA Act is trying to get co-sponsors = that’s the bill to permanently fix PAMA calculations. (ACLA letter on the topic attached) (link)
C. CMS proposed CLIA changes
The proposed CMS CLIA changes would allow people with nursing degrees to perform moderate and high complexity testing (proposal attached).
Groups like ASCP [ascp.org] and AACC (letter attached) are up in arms about that.
MDIC Updates
Successfully Navigating the USPTO and FDA Seminar (link to register)
September 8th
Virtual
Cybersecurity Summit (link to register)
September 12th
Virtual and in-person- JW Marriott Washington DC: 331 Pennsylvania Avenue NW, Washington, District of Columbia, 20004
Annual Public Forum (link to register)
September 13th
JW Marriott- Washington DC: 331 Pennsylvania Avenue NW, Washington, District of Columbia, 20004
5G Communication in Healthcare: Background, Landscape, and Use Cases
Hosted on August 8th
A webinar focused on an overall description of 5G communication technology, the role 5G plays in the healthcare landscape, and examples of 5G-enabled healthcare applications including medical extended reality (MXR), robotics, mobile units, and remote care.
Recording can be found at: https://www.youtube.com/watch?v=9CeoiF7BWew
Societies and Professional Organizations
Digital Pathology Association
Digital Anatomic Pathology Academy (DAPA)
WSI educational platform provided by the DPA for its members
Cloud-based platform which provides annotated digital slides with diagnosis and relevant information of morphology and ancillary testing
Accessible from anywhere, on any device, without downloading any software
World Health Organization Open Positions
https://www.iarc.who.int/vacancy/it-database-and-web-developer-req-2207536/
https://www.iarc.who.int/vacancy/scientist-exposure-req-2207524/
https://www.iarc.who.int/vacancy/scientist-epidemiology-req-2207525/
https://www.iarc.who.int/vacancy/scientist-toxicology-req-2207533/
https://www.iarc.who.int/vacancy/scientist-toxicology-req-2207526/
https://careers.who.int/careersection/ex/jobdetail.ftl?job=2207525
Regulatory Affairs Professionals Society (RAPS)
The Patient-Centered Health Award recognizes organizations or individuals for significantly advancing patient-centered policy, product development or regulatory decision-making. The recipient of this year’s honor is:
Friends of Cancer Research
Friends of Cancer Research Update
Timelime TMB Harmonization Project
ctDNA:
Friends of Cancer Research ctMoniTR Project Presentation
Call for papers: Liquid Biopsies (scientific reports)
cfDNA methylome profiling for detection and subtyping of small cell lung cancers (download)
Detection of Circulating Tumor Cells in Cerebrospinal Fluid of Patients with Suspected Breast Cancer Leptomeningeal Metastases: A Prospective Study (download)
Is liquid biopsy the future commutator of decision-making in liver transplant for hepatocellular carcinoma? (download)
ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer; a report from the ESMO Precision Medicine Working Group (download)
Circulating tumor DNA to guide rechallenge with panitumumab in metastic colorectal cancer: the phase 2 CHRONOS trial (download)
Circulating tumour DNA - looking beyond the blood (download)
Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients with Non-Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors (download)
Longitudinal Undetectable Molecular Residual Disease Defines Potentially Cured Population in Localized Non-Small-Cell Lung Cancer (download)
Barriers to adopting digital pathology in developing economies and mitigation strategy
Diversity and Inclusion
Human Rights Campaign
https://www.hrc.org/resources/workplace
HRC works to provide employers the resources
They need to improve and promote fairness in the workplace
Talking about pronouns in the workplace (download)
Article: 5 Specifications That The FDA's Diversity Plan Needs To Include
In April 2022, the FDA made available for public comment its Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials
Implement A Community Engagement Plan
Be More Inclusive With Your Eligibility Criteria
Provide Resources For Patients To Address/Overcome Barriers To Trial Adherence
Include Sites In Diverse Areas
Take On Accountability For Diversity Plan Adherence
Link to article: https://www.clinicalleader.com/doc/specifications-that-the-fda-s-diversity-plan-needs-to-include-0001
Resources
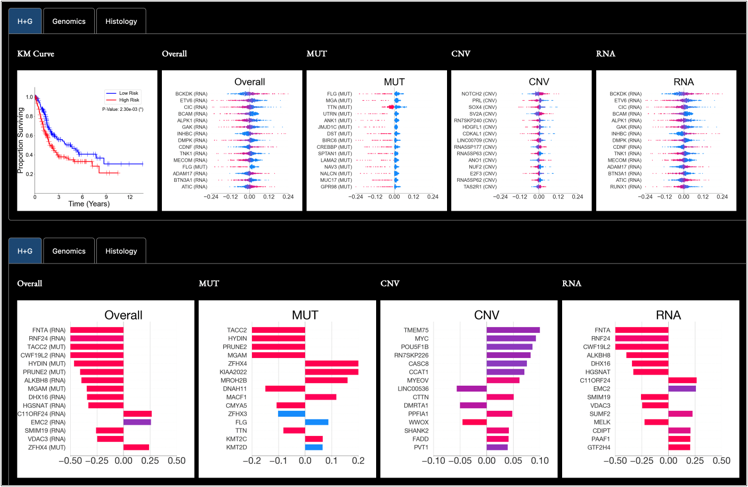
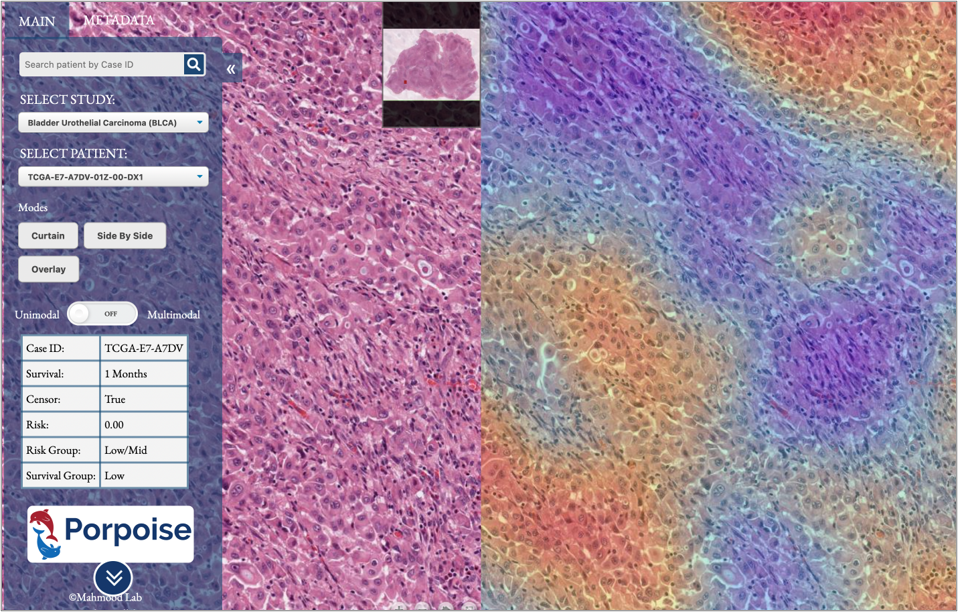
Pathology-Omic Research Platform for Integrated Survival Estimation (link)
ESO-EDP Digital Pathology Seminar is an event devoted to physicians, specifically to pathologists and oncologists, focusing on the digital transformation of the pathology laboratories and consequent benefits. Here, examples of digital workflow implementation will be demonstrated, as well as the potentialities of image analysis tools in the setting of biomarkers and, broadly, in cancer models. (link)
FutureBridge Article: Challenges and Drivers of Translational Research (link)
Broad Institute: rare variant resource. Genebass summarizes a genetic analysis of nearly 400,000 people in the UK Biobank and could help researchers identify new therapeutic targets. (link)
FDA Oncology Center of Excellence – 2021 Annual Report (link)
FDA: Project Socrates (link)
•Episode 1: FDA’s role in Oncology Product Development
•Episode 2: Oncology Trial Design Considerations
•Episode 3: Statistical Considerations in Designing Cancer Clinical Trials
•Episode 4: Investigational New Drug Applications
Guidebook: In order to use this Guidebook effectively, the following areas must be agreed upon by institution and country leadership prior to LIS selection (download):
1.Defining success
2.Defining standards
3.Adopting a standard set of procedures
4.Defining sustainability for the country/laboratory.
Book feature- The Empowered Woman’s Guide to Better Health by Mary I. O’Connor, MD and Kanwal L Haq MS (download)
Medicines and Healthcare Products Regulatory Agency Guidance: Medical device stand-alone software (download)
Publications
Organ Specific
Provencio et al., Overall Survival and Biomaker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial) (download)
Cooper et al., Clinicopathologic characteristics and outcomes for patients with KRAS G12D-mutant non-small cell lung cancer (download)
Margolis et al., Activating IGF1R hotspot non-frameshift insertions define a novel, potentially targetable molecular subtype of adenoid cystic carcinoma (download)
Liu et al., Integrative tumour mutation burden with CD39 and PD-L1 for the prediction of response to PD-L1 blockade and adjuvant chemotherapy in muscle-invasive bladder cancer patients (download)
Pourmaleki et al., Tumor MHC Class I Expression Associates with Intralesional IL2 Response in Melanoma (download)
NSCLC Without Driver Guidance
Singh et al., Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline (download)
CSER Consortium
O’Daniel et al., Integration of stakeholder engagement from development to dissemination in genomic medicine research: Approaches and outcomes from the CSER Consortium (download)
Arrieta et al., Explainable Artificial Intelligence (XAI): Concepts, Taxonomies, Opportunities and Challenges toward Responsible AI (download)
Montesinos-López et al., A review of deep learning applications for genomic selection (download)
Capobianco, High-dimensional role of AI and machine learning in cancer research (download)
Drogt et al., Integrating Artificial Intelligence in pathology: a qualitative interview study of users’ experiences and expectations (download)
Chen et al., Pan-cancer integrative histology-genomic analysis via multimodal deep learning (download)
Lutnick et al., A user-friendly tool for cloud-based whole slide image segmentation with examples from renal histopathology (download)
Marini et al., Unleashing the potential of digital pathology data by training computer-aided diagnosis models without human annotations (download)
Joo et al., Multimodal deep learning models for the prediction of pathologic response to neoadjuvant chemotherapy in breast cancer (download)
Joshi et al., Look alike humans identified by facial recognition algorithms show genetic similarities (download)
Bridge et al., Highdicom: a Python Library for Standardized Encoding of Image Annotations and Machine Learning Model Outputs in Pathology and Radiology (download)
Blanco et al., From Scientific Discovery to Covered Treatments Understanding the Payer Perspective as a Keystone to Achieving High-Value Care (download)
Future Trends in Spatial Biology; the Pathologist
The use of spatial biology in laboratory medicine is on the rise – but what does the future hold?
Automation
Resolution
Multi-omics and multiplex
Artificial Intelligence
Sample quality
Standardized diagnostic biomarkers
Link: https://thepathologist.com/diagnostics/future-trends-in-spatial-biology
Events
August 22 - 24, 2022 Next Generation Dx Summit in Washington, DC (link)
September 21 - 23, 2022 Digital Diagnostic Summit in Park City, Utah (download)
September 9 - 13, 2022 ESMO Conference 2022, Paris (link)
August 26 - 28, 2022 Annual Meeting of Japanese Society of Digital Pathology (link)
October 27, 2022 OSU Digital Pathology Workshop (link)
Inside the Lab Podcast with Sanjay Mukhopadhyay (link)
August 10, 2022 Tian Yu: Future Trends in Spatial Biology; the Pathologist (link)