HER2-Low Project
In 2022 a new therapy became available for a significant subset of breast cancer patients.
There are currently nearly 300,000 new cases diagnosed per year in the US. Approximately 80-85% of those new cases were previously considered to be HER2-negative subtype (including hormone receptor positive and triple negative breast cancer), which means the tumors do not overexpress, or make too many copies of the HER2 protein. Of that proportion of breast cancer diagnoses, about 60% of patients previously classified as having HER2-negative subtype can now be considered as HER2-low. HER2-low patients received endocrine therapy or chemotherapy. Patients with so-called HER2-low breast cancer are now eligible for a certain therapy if they have received a prior chemotherapy in the metastatic setting (or their cancer returned during, or within 6 months of completing, adjuvant chemotherapy).
In this project, we are outlining regulatory aspects of the diagnostic assays and relevant aspects for realization.
HER2-Low Project Session I
April 7th 2023
In the first scoping meeting, we covered a broad range of relevant topics. The scoping session has resulted in an overview of relevant themes. Watch a recording of the session here.
We ask all project members and PIcc participants to vote for the subtopic that they find most relevant (e.g. for a regulatory science project).
If you have additional publications, resources, or links, please provide those.
Project Team
Please reach out to digipathalliance@gmail.com or join the next project session (5/12 at 12-1PM EDT) if you’d like to contribute.
HER2-Low Project Session II
May 12th 2023
Resources
Stuart J. Schnitt, Paolo Tarantino, & Laura C. Collins. The American Society of Clinical Oncology–College of American Pathologists Guideline Update for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: How Low Can HER2 Go? (download .pdf)
SWOT analysis
Baez-Navarro et al., Selecting patients with HER2-low breast cancer: Getting out of the tangle
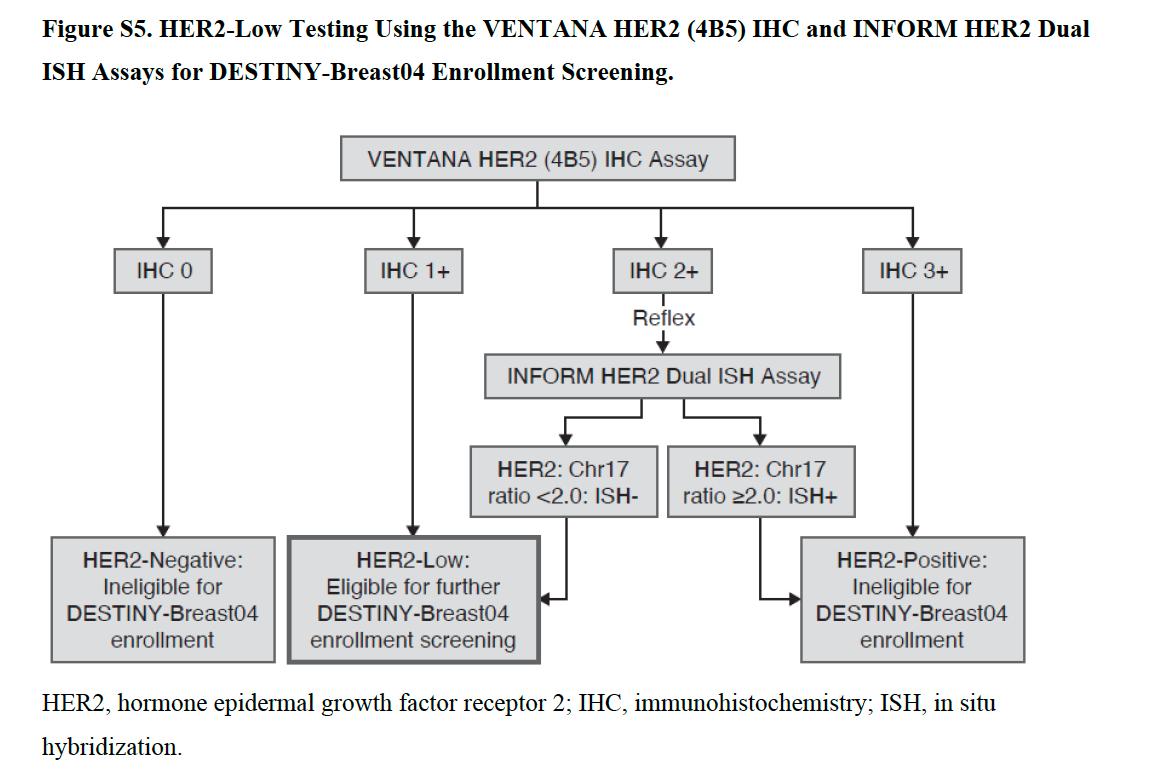
Study by Modi et al., NEJM. DESTINY-Breast04 trial
In this trial involving patients with HER2-low metastatic breast cancer, trastuzumab deruxtecan resulted in significantly longer progression-free and overall survival than the physician’s choice of chemotherapy.
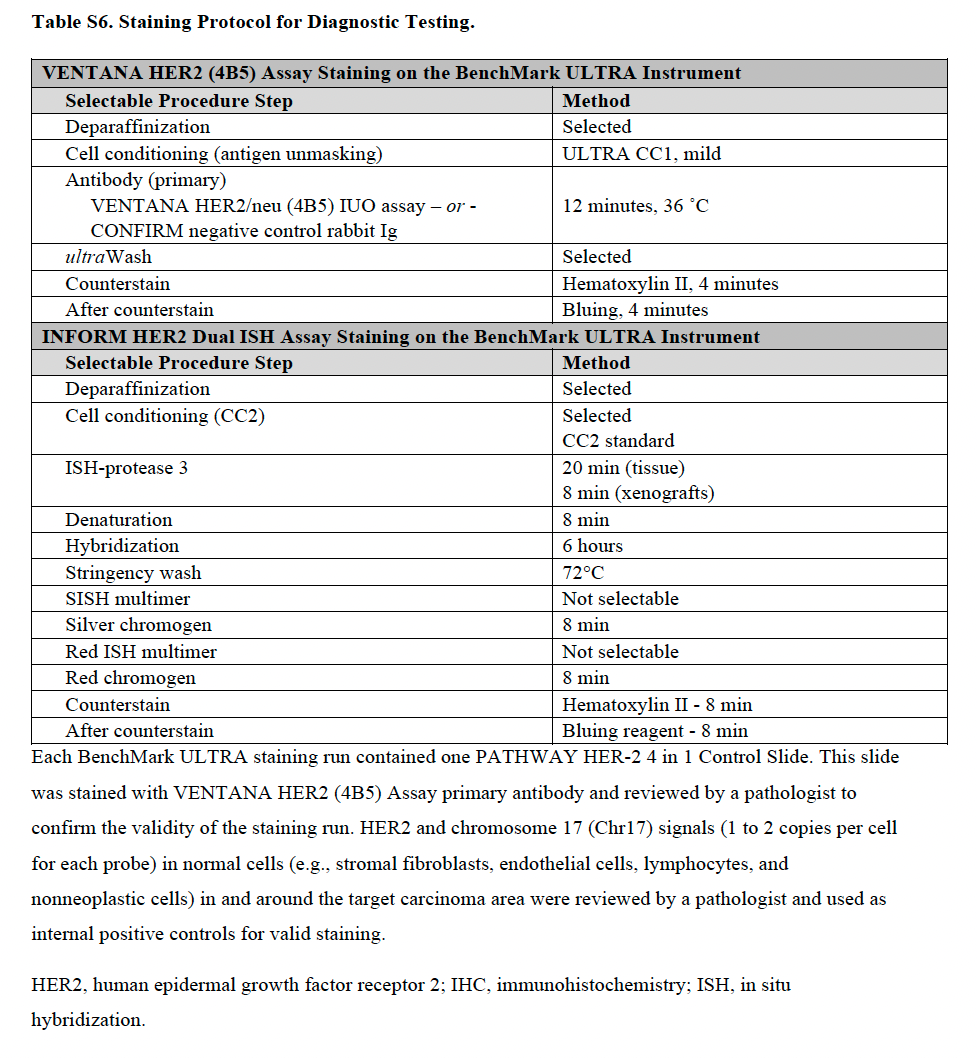
Staining protocol for diagnostic testing
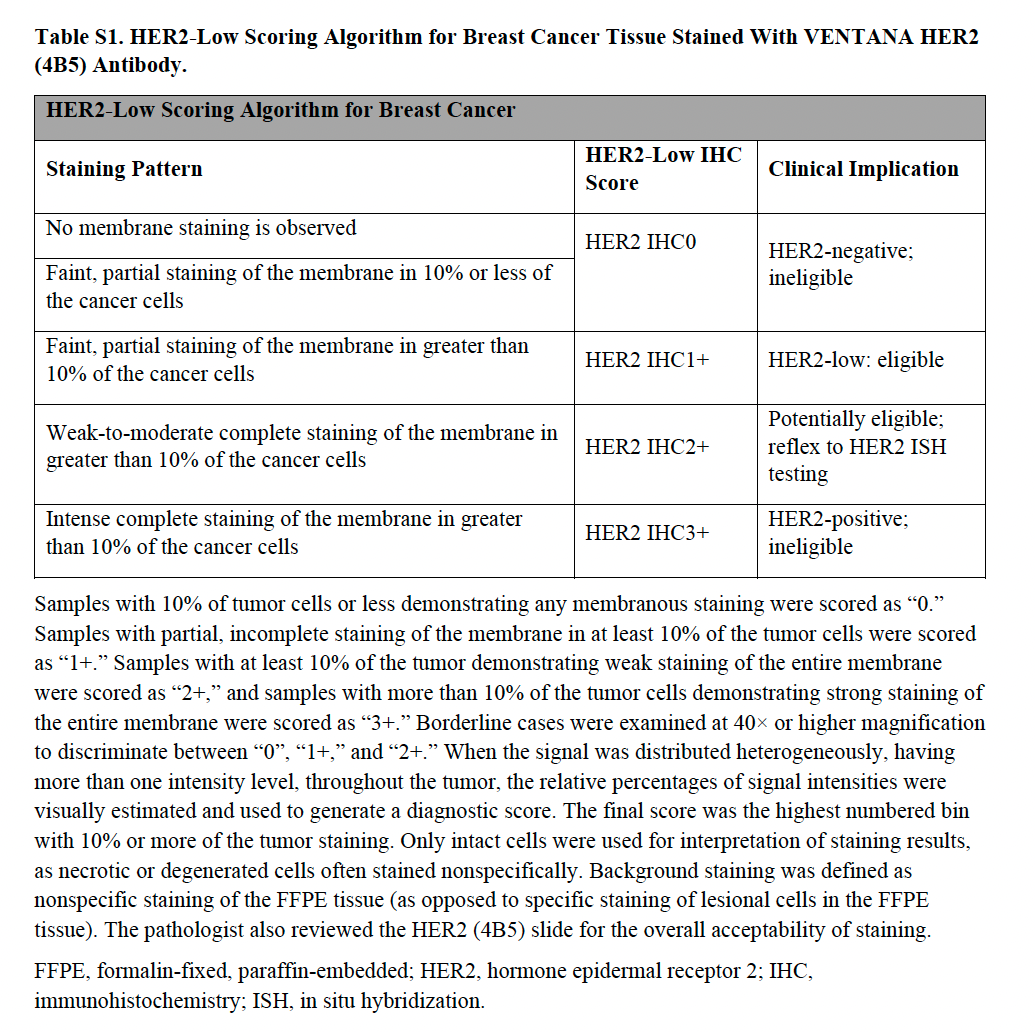
HER2-Low Scoring Algorithm for Breast Cancer Tissue Stained With VENTANA HERZ(4B5) Antibody
HER2 scoring recommendations
Paper: Andre et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update (download PDF)
Paper: Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update (download PDF)
Example: FISH
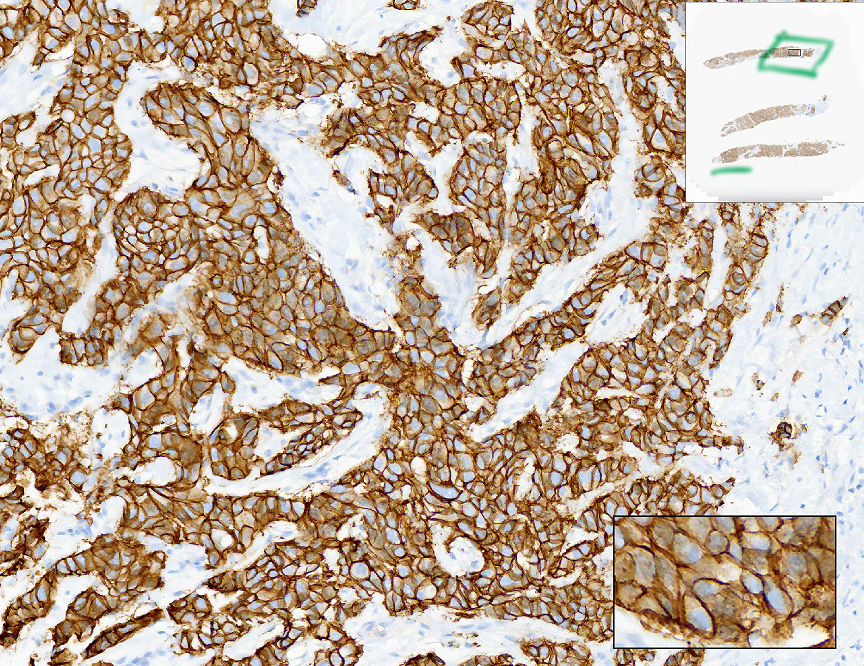
Example: 3+
Ardor, Komforti & Nassar. Evaluating Low HER2 Status in Invasive Breast Carcinoma via HER2 Immunohistochemistry, a Cohort of 112 Patients
Currently Approved Assays
Publications
Boyraz et al., Discerning subsets of breast cancer with very low and absent HER2 protein expression (download PDF)
Xu et al., Discordance between Immunohistochemistry and Erb-B2 Receptor Tyrosine Kinase 2 mRNA to Determine Human Epidermal Growth Factor Receptor 2 Low Status for Breast Cancer (download PDF)
Tarantino et al., Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer (download PDF)
Bilous et al. Current Perspectives on HER2 Testing: A Review of National Testing Guidelines (link)
Steiner et al. Evaluation of the Use of Combined Artificial Intelligence and Pathologist Assessment to Review and Grade Prostate Biopsies (link)