PCCP Project
Project Session I - May 3
April 13, 2023
FDA hosted a webinar discussing the recently released draft guidance. The webinar provided background on FDA’s patient-centered approach, scope of the guidance, modifications for ML-DSFs, and provided examples.
Access additional resources via CDRH Learn, Specialty Technical Topics
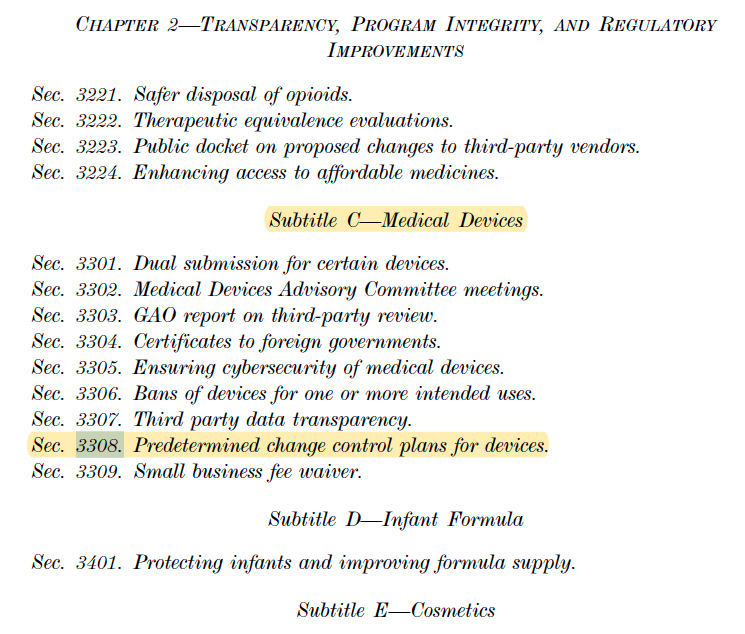
Sec. 3308 of the Consolidated Appropriations Act 2023 includes
Predetermined Change Control Plans for devices: If a predetermined change control plan is approved or cleared, then a supplemental PMA or a new 510(k) is not required for a change to a device that is consistent with such approved or cleared plan
Update March 30, 2023:
CDRH Issues Draft Guidance on Predetermined Change Control Plans for Artificial Intelligence/Machine Learning-Enabled Medical Devices (link)
Download the draft guidance (link) (download .pdf)
Submit comments online through 7/3/2023 (link)
HHS Announcement (download .pdf)
Summary: As indicated by Sec. 3308 of the Consolidated Appropriations Act 2023 this is the subsequent guidance defining the approach to regulate predetermined change control plans. The document includes Predetermined Change Control Plan (PCCP), Authorized Predetermined Change Control Plan (Authorized PCCP), Modification Protocol, as well as Impact Assessment.
We will host a session to review the draft guidance.
Background
One of the key elements in the proposed regulatory approach to AI/ML is the so-called Predetermined Change Control Plan (PCCP).
A PCCP allows manufacturers to specify the types of anticipated modifications for the software and the associated methodology being used to implement those changes
The PCCP framework will likely entail that a manufacturer defines and submits a PCCP to the FDA in a premarket submission. Under the anticipated framework, the manufacturer can then use the PCCP to make modifications that are within the bounds of the PCCP
Aims of the Project
Highly relevant for developing a proposed regulatory framework - including through issuance of draft guidance on a predetermined change control plan (for software’s learning over time);
We anticipate guidance to follow quickly.
We will host a session/project on Pre-determined Change Control Plan.
Aim is to
capture the relevant regulatory information
review PCCP
establish a resource with the relevant context when the draft guidance goes live
Resources
Appropriations Bill (download PDF)
George et al., A First Look at the FDA’s Proposed Regulatory Framework for Modifications to AI-Based Software as a Medical Device (SaMD): IP Review and Strategy Guide (download PDF)
FDA: Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) - Discussion Paper and Request for Feedback (download PDF)