3/29/2023 Updates Meeting
Steering Committee Series
Watch the recording of the meeting here.
FDA
Center for Drug Evaluation and Research (CDER):
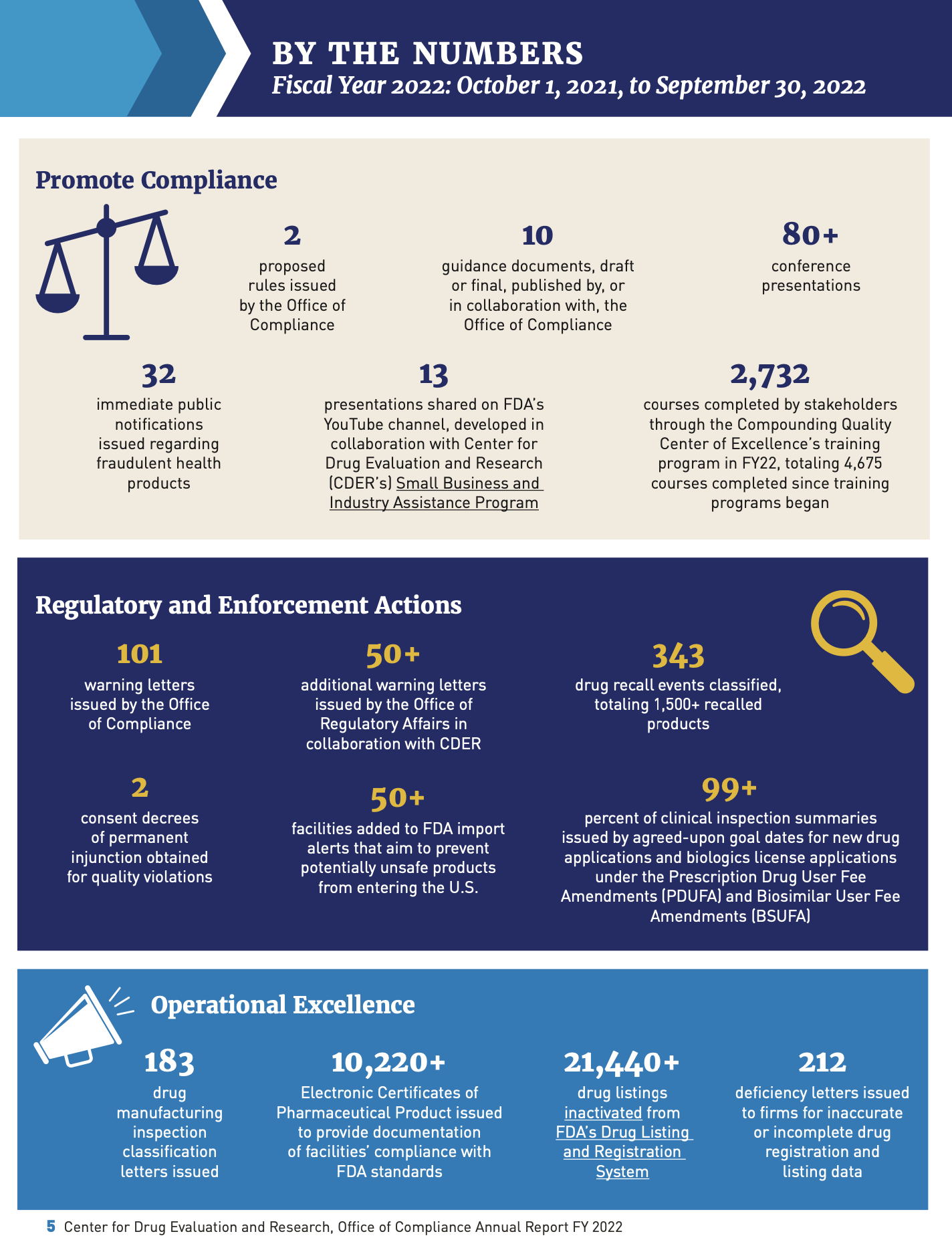
Office of Compliance Annual Report Fiscal Year 2022 (download .pdf)
Artificial Intelligence in Drug Manufacturing (download .pdf)
Guidance for Industry:
Q13 Continuous Manufacturing of Drug Substances and Drug Products (download .pdf)
Updates:
Learn:
Training Course: Achieving Data Quality and Integrity in Maximum Containment Laboratories (link)
Webinar on Guidances on COVID-19 Transition Plans for Medical Devices (link)
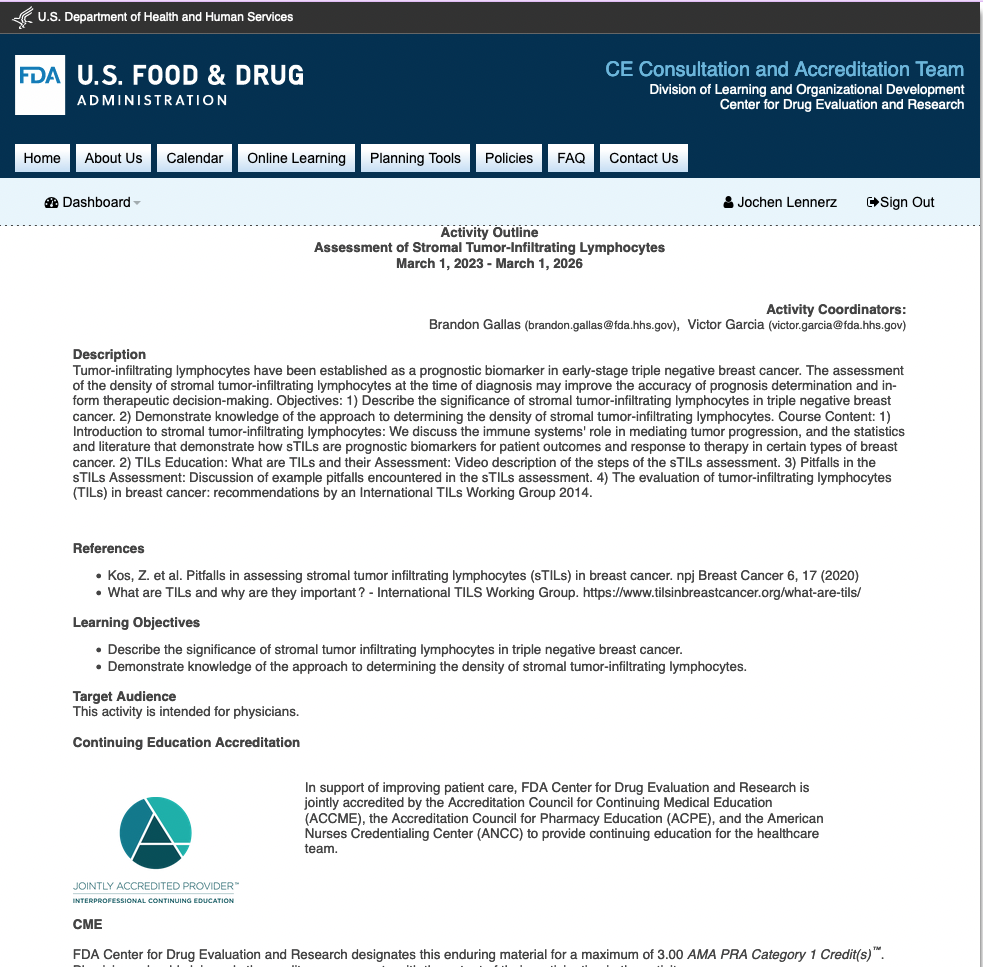
Continuing Medial Education (CME): Assessment of Stromal Tumor-Infiltrating Lymphocytes (link to CE Portal) (NCI link)
The FDA-led High Throughput Truthing (HTT) project created a 3.00 Credit Hour CME Course titled the Assessment of Stromal-Tumor Infiltrating Lymphocytes. Hosted on the FDA’s Continuing Education (CE) portal, the course introduces the clinical significance of stromal tumor-infiltrating lymphocytes (sTILs), gives an overview of how to perform and identify pitfalls in the sTILs assessment, and includes a manuscript by Salgado et al. (Annal Oncol., 2015) on TILs assessment recommendations by the International Immuno-Oncology Biomarker Working Group.
download the relevant Salgado et al. paper: The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014
download the relevant Garcia et al. paper: Reference Material for the Assessment of Stromal Tumor-Infiltrating Lymphocytes in the High Throughput Truthing Project
download the 4-part course slides
News
update 3/30/23: CDRH Issues Draft Guidance on Predetermined Change Control Plans for Artificial Intelligence/Machine Learning-Enabled Medical Devices (link to PCCP project for more info)
Federal Register: Guidance Documents Related to Coronavirus Disease 2019 (COVID–19)
Final FDA Guidance: Transition Plan for Medical Devices That Fall Within Enforcement Policies Issued During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (download .pdf) (link)
For an overview of the most recent updates on changes coming to remote work, view these slides.
LDT
Download the brief overview slides here
VALID to be reintroduced: Rep. Larry Bucshon plans to reintroduce the bill Wednesday afternoon with Rep. Diana DeGette (link)
View the update bill (download .pdf)
Congress Holds Off on Enabling FDA Regulation of Clinical Laboratory-Developed Tests (link)
FDA Resumes Move to Regulate LDTs, Likely Setting up Legal Battle With Lab Industry (link)
DEI
Podcast - Women in Pathology Informatics: A Conversation with CAP Staffer Mary Kennedy (link)
Mary was asked to do a podcast on the CAP app on women in informatics and what it means. The point is to try to persuade women pathologists to become more engaged with informatics and all that it encompasses
The Legacy of Mary Kenneth Keller, First U.S. Ph.D. in Computer Science (download .pdf)
Government
Arkansas State Legislature: HB1121 - CONCERNING COVERAGE FOR BIOMARKER TESTING FOR EARLY DETECTION AND MANAGEMENT FOR CANCER DIAGNOSES (link)
White House Fact Sheet: Biden-Harris Administration Announces New Bold Goals and Priorities to Advance American Biotechnology and Biomanufacturing (link)
The White House Office of Science and Technology Policy: Bold Goals for U.S. Biotechnology and Biomanufacturing (download .pdf)
New York State Approves AI-Based Diagnostic Test For Breast Cancer (link)
CMS
CMS to work closely with FDA on accelerated approval payment reforms (link)
CMS held a two day workshop on "coverage with evidence development." (link)
AI prior authorization (link)
Electronic Prior Authorization for Prescription Drugs — Challenges and Opportunities for Reform (link)
Resource AI (link)
MDIC
PIcc23: in-person workshop June 27 & 28 in the D.C. area
more details coming soon
MDIC Live Fireside-Chat style conversation with MDIC Leadership (link)
Join MDIC on March 30 for an informational webinar on the Medical Device Computational Modeling and Simulation Landscape Report (link)
Call for Volunteers! MDIC Digital Health Software Vertical
For more information, please contact: Jithesh Veetil jveetil@mdic.org or Taylor Matheny TMetheny@mdic.org
Seeking Subject Matter Expert volunteers to support Science of Patient Input Post-Market Patient Engagement Working Groups
Leadership Engagement Culture Initiative
Interested? Contact cfqcc@mdic.org to get involved with Case for Quality initiatives
Resources
Global Pathology Workforce
CADTH Horizon Scan 2023 Watch List: Top 10 Precision Medicine Technologies and Issues (download .pdf)
Coalition for Health AI (CHAI) Blueprint for Trustworthy AI Implementation Guidance and Assurance for Healthcare (download .pdf)
A Road Map For Action: Recommendations Of The Health Affairs Council On Health Care Spending And Value (download .pdf) and Executive Summary(download .pdf)
Longbing Cao. Data Science: A Comprehensive Overview (download .pdf)
Global pathology workforce heat map (link)
Cancer Moonshot colorectal cancer forum (link)
Survival Models for Histopathology: A review of machine learning techniques for predicting patient outcomes from whole slide images (link)
Publications
Europe PMC Annotated Full-text Corpus for bioentities and associations: “To facilitate information discovery and foster literature–data integration, Europe PMC has incorporated text-mining approaches into its workflows.” (link to update) (link to tool)
SciLite Annotations (link to update)
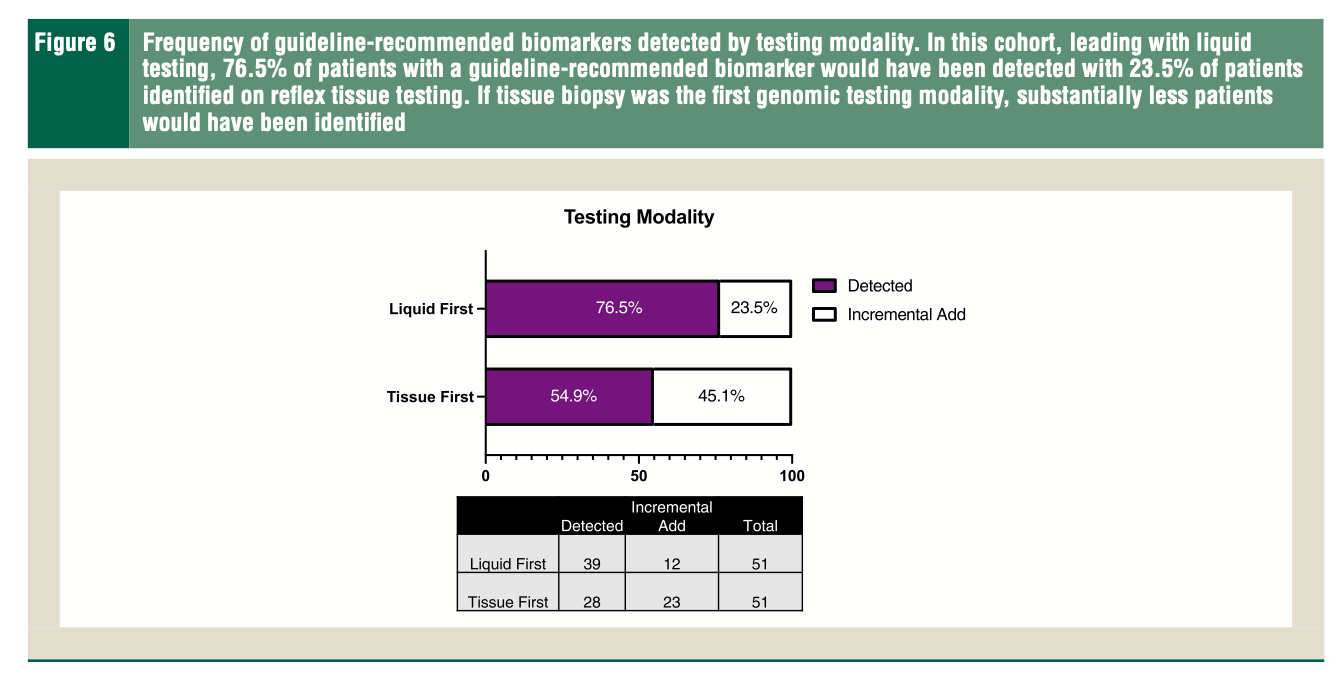
Raez et al. Liquid Biopsy Versus Tissue Biopsy to Determine Front Line Therapy in Metastatic Non-Small Cell Lung Cancer (NSCLC) (download .pdf)
#non-small cell lung cancer #biopsy #tumor
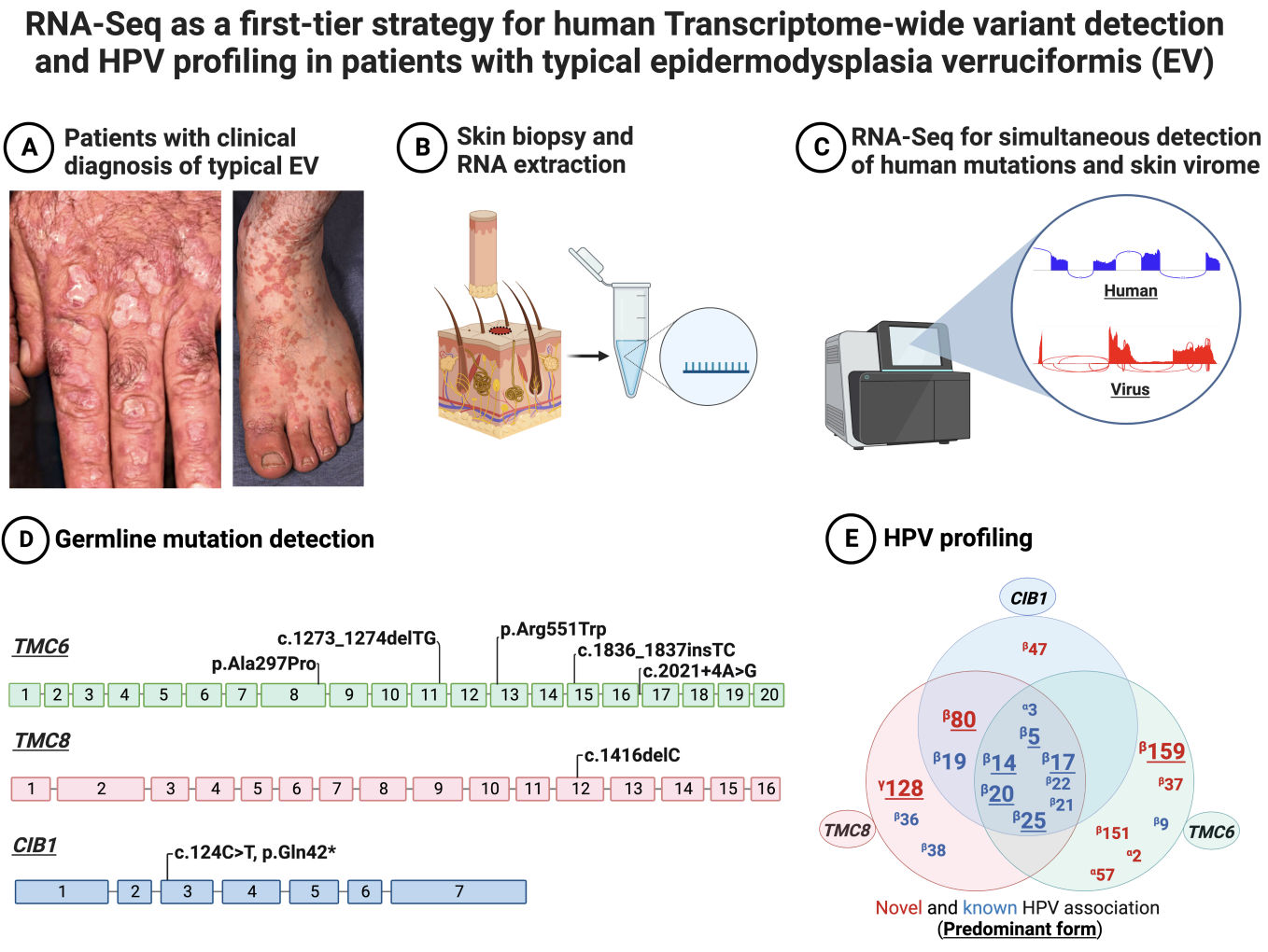
Saeidian et al. Whole transcriptome-based skin virome profiling in typical epidermodysplasia verruciformis reveals α-, β-, and γ-HPV infections (download .pdf)
#skin cancer #pathogenesis #TMC6 #TMC8 #CIB1 #HPV-2
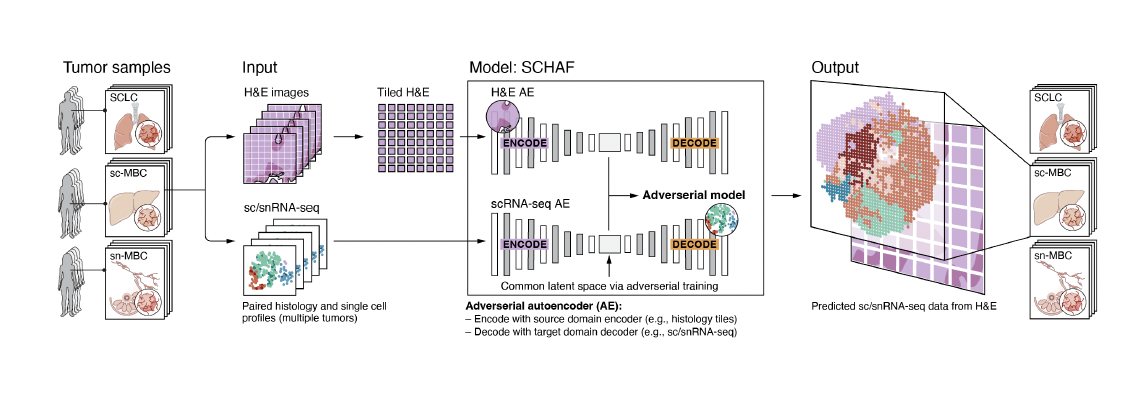
Comiter et al. Inference of single cell profiles from histology stains with the Single-Cell omics from Histology Analysis Framework (SCHAF) (download .pdf)
#RNA-seq #metastic breast cancer #scRNA-seq #MERFISH #tumor #learning
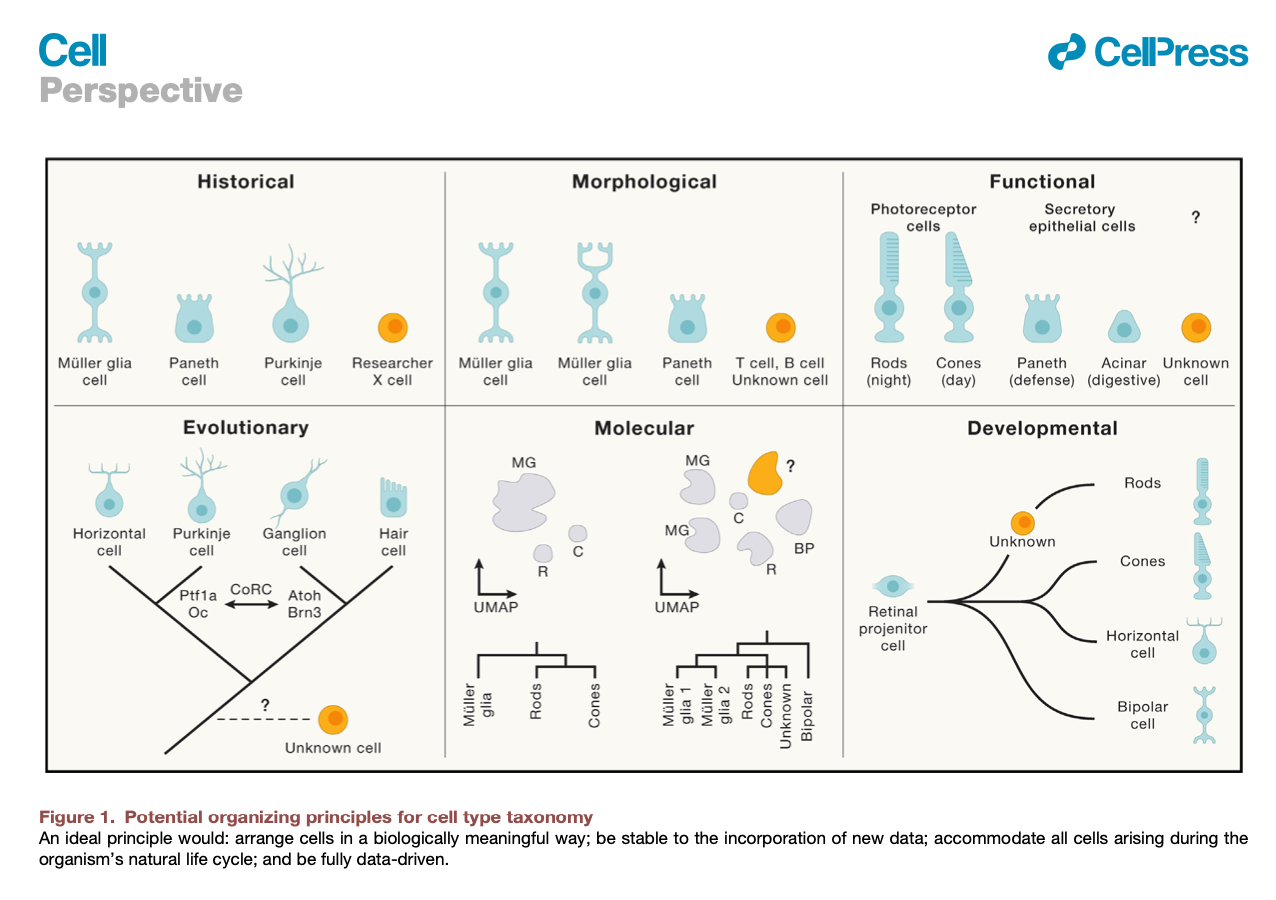
Domcke & Shendure. A reference cell tree will serve science better than a reference cell atlas (download .pdf)
#cell atlas #classification #ontogeny
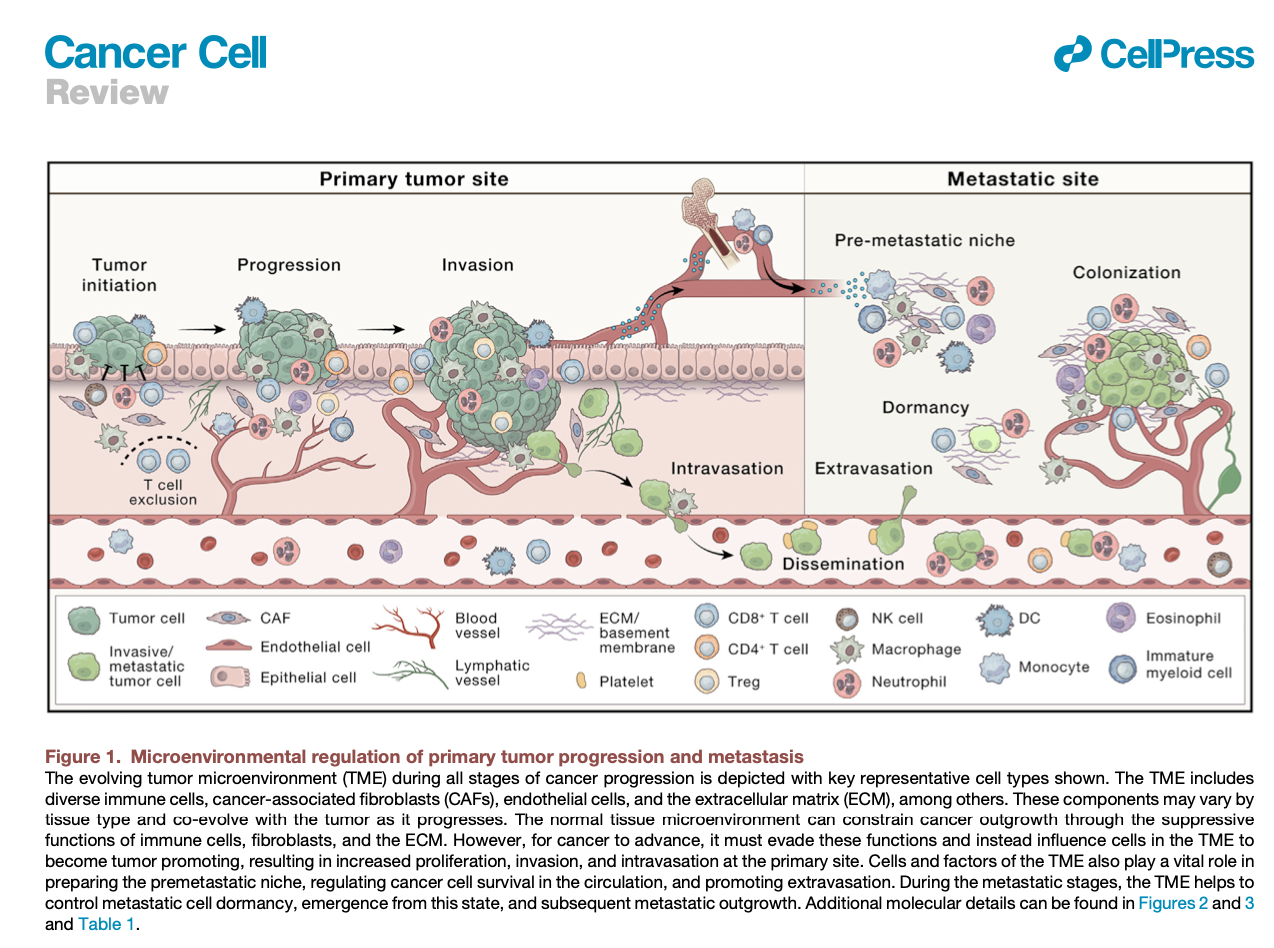
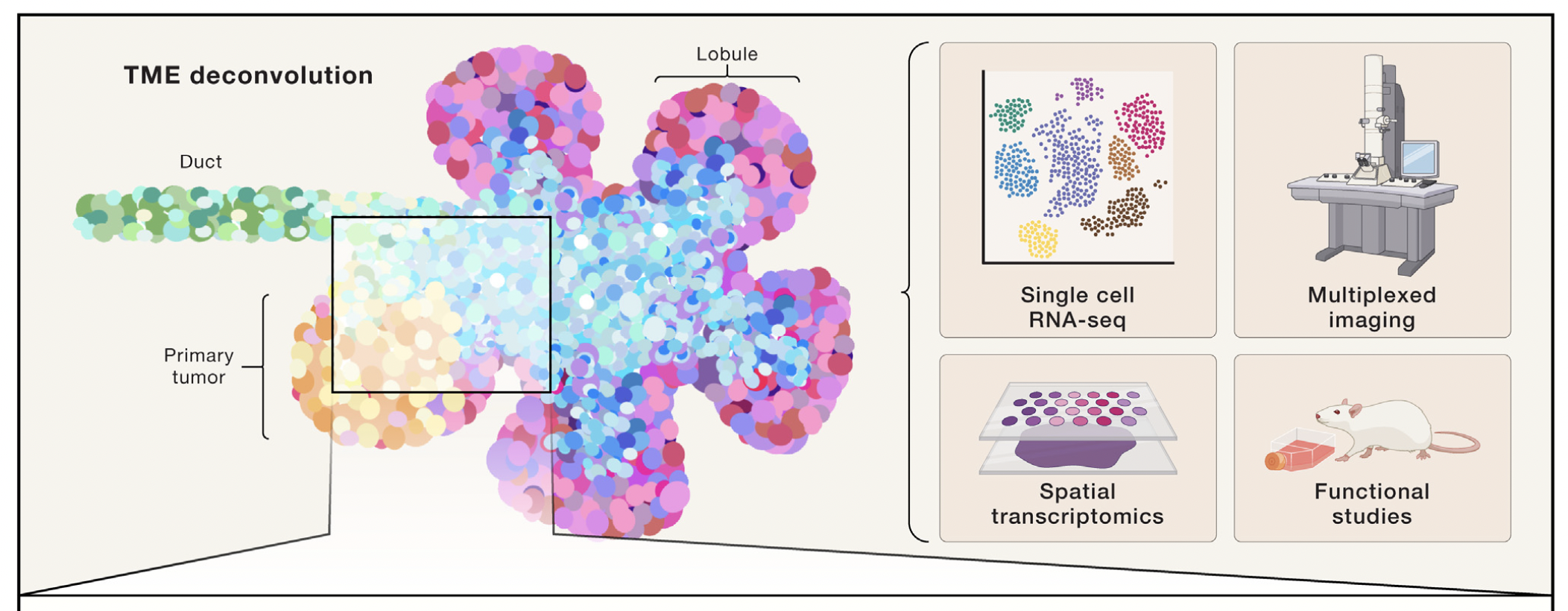
de VIsser & Joyce. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth (download .pdf)
#tumor #cancer progression #tumorigenesis #cancer #pathogenesis #extracellular matrix
L’Imperio et al. Pathologist Validation of a Machine Learning–Derived Feature for Colon Cancer Risk Stratification (download .pdf)
#learning #TAF #MMR #MSI #BRAF #tumor #colorectal cancer #mismatch repair
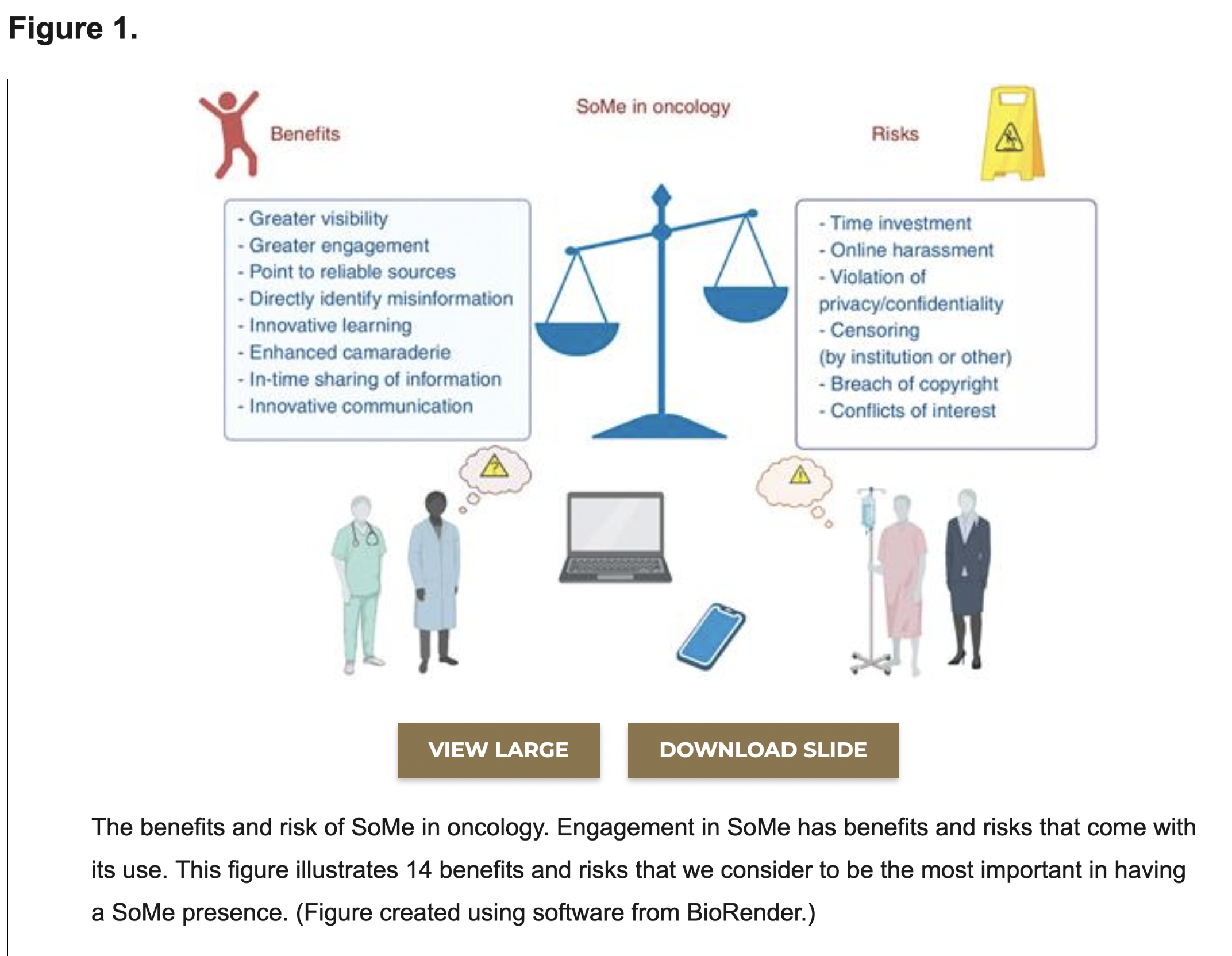
Morgan et al. The (R)evolution of Social Media in Oncology: Engage, Enlighten, and Encourage (download .pdf)
#cancer
Nolan, Lindeman, & Visvader. Deciphering breast cancer: from biology to the clinic (download .pdf)
#cancer
Shinohara et al. Substantial improvement of histopathological diagnosis by whole‐slide image‐based remote consultation (download .pdf)
#cancer #PBC #AIH #hyperplastic #papillary thyroid carcinoma
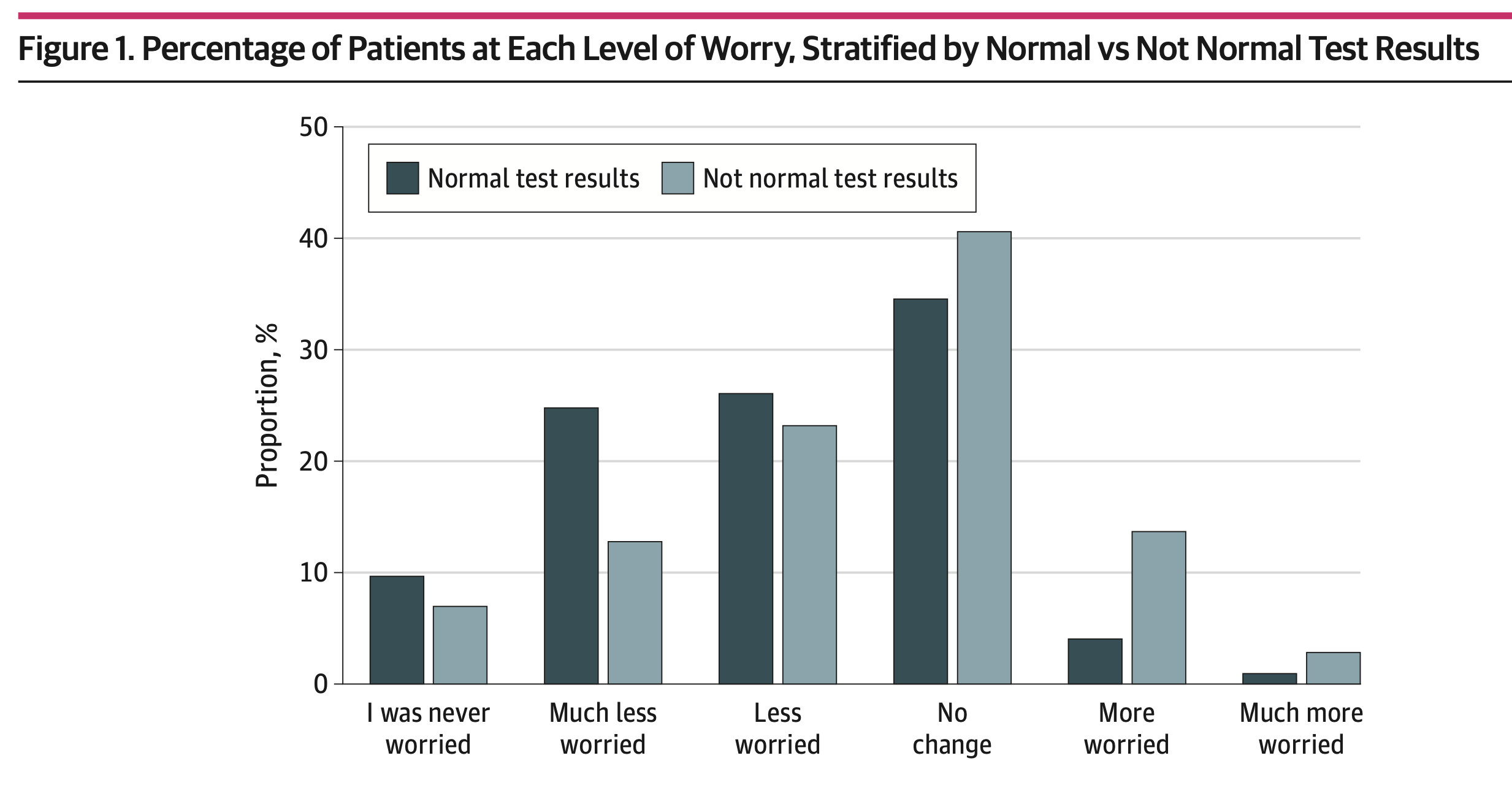
Steitz et al. Perspectives of Patients About Immediate Access to Test Results Through an Online Patient Portal (download .pdf)
#behavior #emotional distress #depression #biopsy #tumor
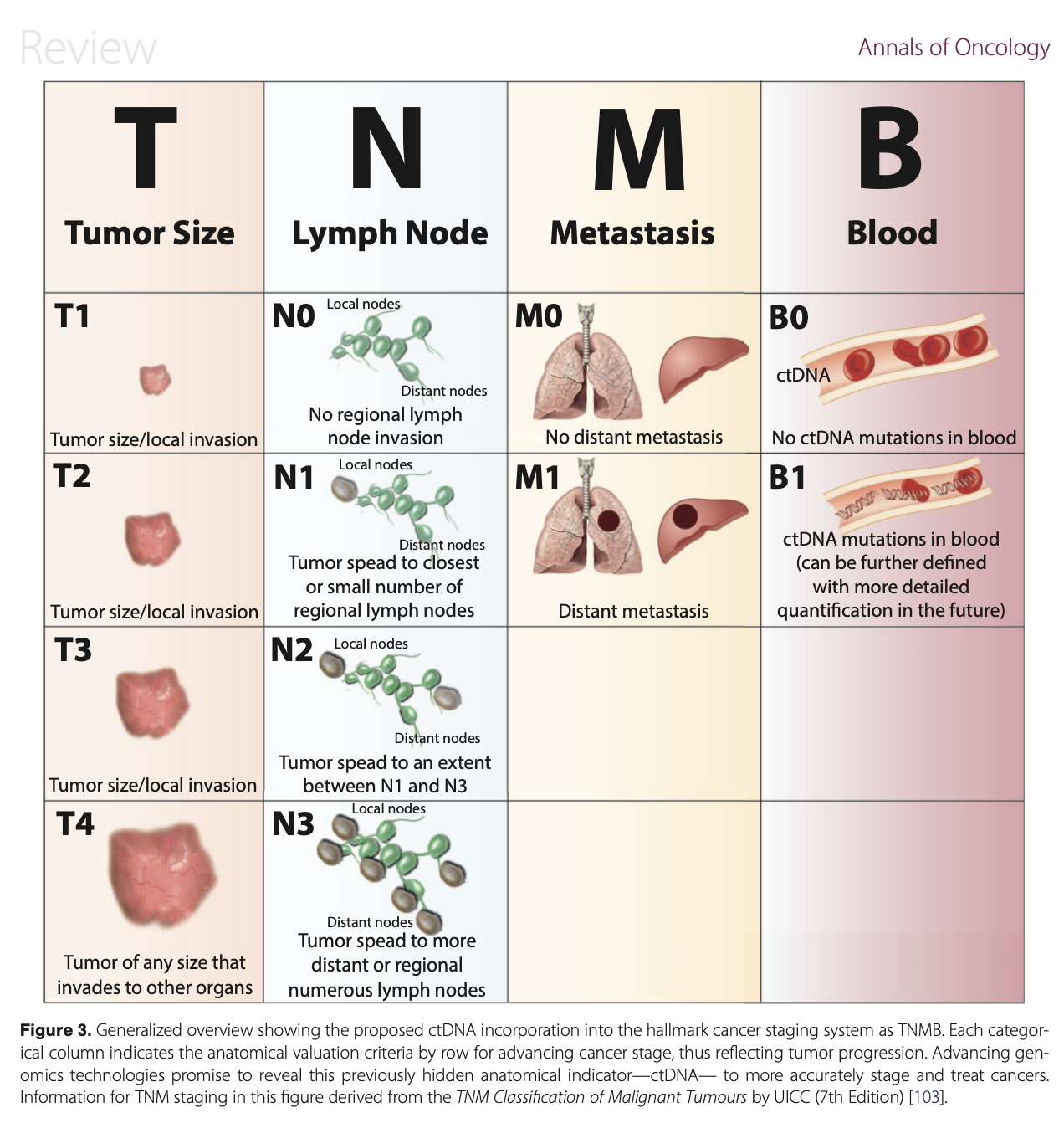
Yang et al. Incorporating blood-based liquid biopsy information into cancer staging: time for a TNMB system? (download .pdf)
#cancer #tumor #EGFR #KRAS #GRAIL #BRAF #clonal evolution #tumor progression
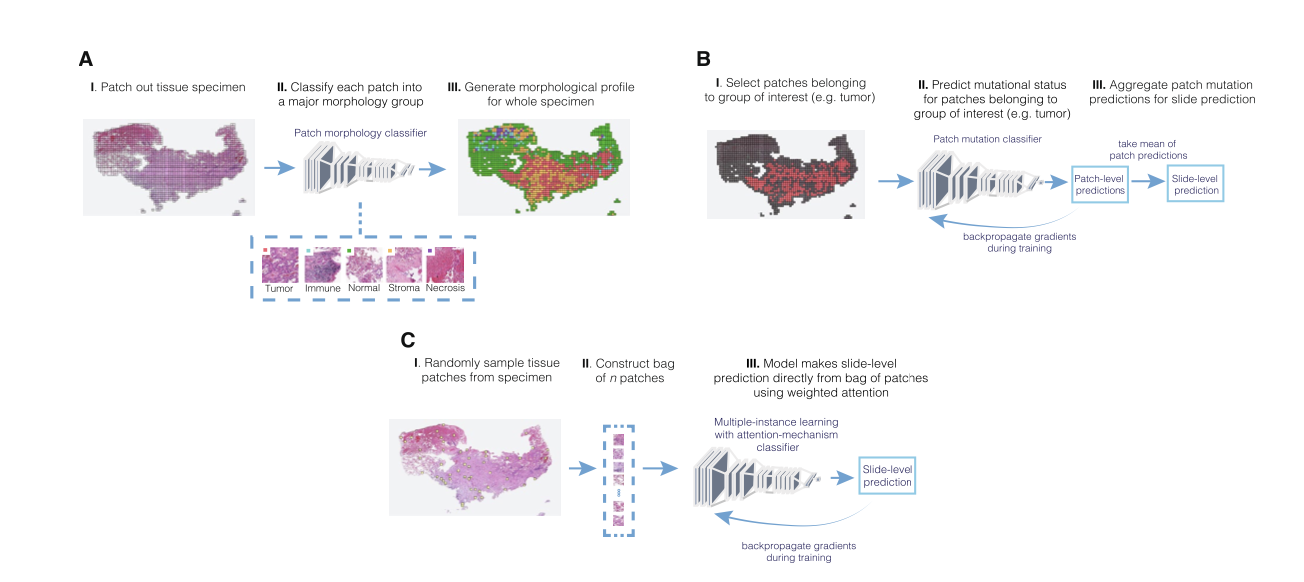
Pao et al. Predicting EGFR mutational status from pathology images using a real‐world dataset (download .pdf)
#learning #EGFR #MIL #tumor #lung adenocrcinoma #cancer #PPV #immune response #tumor #non-neoplastic